Primary extranodal B-cell lymphoma: current concepts and treatment strategies
Introduction
Approximately one-third of non-Hodgkin lymphomas (NHL) arise from sites other than lymph nodes, spleen or the bone marrow. They may also arise from sites normally devoid of lymphocytes (1,2). Over the last decade, the International Extranodal Lymphoma Study Group (IELSG) has originated several retrospective and prospective trials aimed at clarifying the specific features of primary extranodal NHL (http://www.ielsg.org). However, the majority of published reports remains limited to single-institution retrospective reviews, while, in prospective trials, extranodal lymphomas are usually accounted as nodal lymphomas.
In literature, the definition of primary extranodal lymphomas is still controversial and this may contribute to explain their varying percentage in comparison with the nodal ones (3-5). The designation of stage III and IV lymphomas as primary extranodal NHLs is indeed questionable since extranodal involvement in the presence of mainly nodal or disseminated disease may represent secondary extranodal disease spread. Currently, it is accepted to operationally define as extranodal those lymphomas with no or only “minor” nodal involvement associated with a clinically dominant extranodal component (6). As for the definition, there is no consensus about the staging of primary extranodal lymphomas: the Ann Arbor staging system is at present widely used for describing the extent of the disease. However, specific sites of extranodal lymphoma involvement may require additional work-up procedures (Table 1).
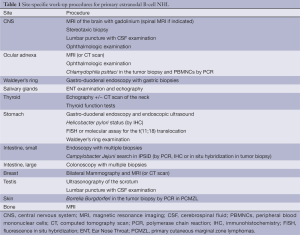
Full table
In the population-based cancer registries of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program, referring to the period 1978-1995 approximately 30% of all lymphomas were extranodal and almost half of all extranodal NHL cases reported had diffuse large B-cell lymphoma (DLBCL) histology (7). The incidence of extranodal NHLs mirrors that of other lymphomas: in countries with high NHL incidence, extranodal disease is also elevated. A great geographic variability of the overall frequency of the extranodal presentation as well as the distribution across the various anatomic sites of onset has in fact been described (8).
Extranodal lymphomas can originate in almost every organ. Data from large series reported in the literature have shown gastrointestinal (GI) tract, skin, bone, and brain to be the most common sites of extranodal lymphoma (6,7,9). While the Ann Arbor staging system considers tonsils and the Waldeyer’s ring as lymphatic localizations, there is controversy about their designation as extranodal sites. Nevertheless, when they are included in the extranodal lymphoma series, the head and neck localizations are the second most frequent site. Moreover, the incidence of primary extranodal presentation is variable across the different B-cell histologic subtypes, encompassing the majority of Burkitt’s lymphomas (BL), up to 50% of DLBCL and less than 10% of follicular lymphomas (FL) (7,8). The distribution of histologic types may be site-specific for some localization such as testis or central nervous system (CNS), where nearly all cases are DLBCL. Conversely, in the GI tract a wide spectrum of lymphoma types can be found, comprising DLBCL, marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT), BL, mantle cell lymphoma (MCL), and FL (6,7,10).
The histologic type is the most important predictor of prognosis in extranodal lymphomas. However, the specific presenting site can also have prognostic implication, and some localization (i.e., the brain and the testis) may require site-specific therapeutic approaches.
GI lymphomas
The GI tract is the most frequently involved extranodal localization, accounting for 30-40% of extra-nodal lymphoma (10-12) and from 4-20% of all NHL cases (5,13). In Western countries, the most common localization is the stomach (approximately 50-60%), followed by the small (30%) and large intestine (around 10%).
Different histological subtypes of lymphoma can arise in the GI tract. The most common histologic subtype in localized GI presentations is DLBCL, which is present in approximately 60% of gastric and 70% of intestinal cases. MALT lymphoma represents about 35% of primary gastric lymphoma and less than 10% of the intestinal ones. FL is very rare in the stomach, but it has been reported in up to 17% of intestinal cases. BL and MCL account globally for about 5% of the cases, the latter often presenting features of multiple lymphomatous polyposis (14,15).
The diagnosis is usually made by endoscopic examination with biopsy and surgery is not needed. Endoscopic ultrasonography is useful in assessing the depth of the wall infiltration in gastric lymphomas, and the presence of perigastric lymph nodes. The usefulness of PET scan has been clearly documented only for DLBCL. Signs and symptoms at presentation are related to the site of origin, regardless of the histologic subtype (abdominal pain, dyspepsia, nausea and vomiting, anorexia, obstruction, hemorrhage). Fever and night sweats are usually absent. Weight loss, however, is common, probably as a consequence of the localization rather than a constitutional symptom of the disease (16).
Primary gastric NHL
Gastric extranodal marginal zone lymphoma of MALT type
MALT lymphoma represents around 7% of all NHL in the Western world. At least one-third of them are a primary gastric lymphoma, which in approximately two-thirds of the cases is associated with a chronic Helicobacter pylori (H. pylori) infection (17). The most common symptoms at presentation are non-specific upper GI disturbances, often leading to an endoscopy, which usually reveals non-specific gastritis or peptic ulcer with mass lesions being unusual (18). Gastro-intestinal bleeding may occur at presentation in 20-30% of patients, while gastric occlusion and perforation are quite infrequent (14). Bone marrow involvement or elevated lactate dehydrogenase (LDH) serum levels are rare in gastric lymphomas (19).
Eradication of H. pylori with antibiotics should be the first-line therapy for a localized H. pylori-positive gastric MALT lymphoma, where the successful eradication can induce lymphoma regression and long-term disease control in most patients (17). Response to eradicative treatment should be checked 6 weeks after the end of antibiotics, and in case of eradication failure, a second-line therapy should be tried with a different regimen (20). While usually a histologically confirmed lymphoma remission is achieved within 6 months, it can also take more than 12 months. Thus, even if histological residual lymphoma persists, in patients obtaining a successful eradication together with a clinical and endoscopic remission it seems appropriate to wait at least 12 months before starting another treatment (17,21). Although there are no uniform criteria yet (17,21), we recommend the GELA (Group d’Etude des Lymphomes de l’Adult) scoring system as a reproducible method to assess the histologic response (17,21,22). After H. pylori eradication, it is recommended to repeat esophagogastroduodenoscopy (EGD) with multiple biopsies 2 to 3 months after treatment to exclude lymphoma progression, and subsequently (every 6 months for 2 years) to monitor the histologic lymphoma regression (20). Notably, the incidence of gastric adenocarcinoma among individual with gastric MALT lymphoma has been reported to be 6-fold higher, the risk of developing other NHL seems also high, while the frequency of high grade transformation seems to be low (23,24). This is an argument for considering long-term endoscopic follow-up of these patients.
There are no definite guidelines for the management of patients with H. pylori-negative gastric-lymphoma and for those failing anti-helicobacter treatments. In H. pylori-negative cases, a regression of the lymphoma after antibiotic treatment is unlikely and the immediate start of oncological treatments should be considered. A standard anti-lymphoma treatment has to be also considered when no lymphoma regression is obtained at an endoscopy evaluation two to three months after antibiotics (25,26). Involved-field radiotherapy (IF-RT) may be the favored choice for patients with H. pylori-negative localized disease or for those failing antibiotic therapy (27). IF-RT with moderate dose (24-30 Gy) to the stomach and perigastric lymph nodes has become a standard treatment, at least in North America. It allows a high rate of local control in MALT lymphoma, with a high proportion of patients likely to be cured (28-32). Thanks to modern techniques, the toxicity to surrounding organs is considerably reduced (28,33,34) and the curative doses (25-35 Gy) are generally associated with mild and reversible acute toxicity and a low risk of long-term side effects (28,34,35).
Patients with symptomatic disseminated disease should also be considered for systemic treatment, not differently from those with other indolent lymphomas. Only a limited number of drugs and regimens have been specifically tested in MALT lymphomas (17), therefore the enrollment in controlled clinical trials seems advisable. Chemotherapy alone, such as alkylating or purine-analogues agents as well as rituximab alone, has been demonstrated to be active. The efficacy and safety of the combinatory treatment with chlorambucil and rituximab has been recently tested by the IELSG demonstrating a significant improvement of complete remission (CR), progression-free survival (PFS) and event-free survival (EFS) rates compared to chlorambucil alone. Nevertheless, no benefit on overall survival (OS) was shown (36,37). The combination of rituximab and bendamustine as well as the combination of fludarabine and rituximab has also shown high rates of disease control in smaller non-randomized studies. Nonetheless, particularly with the latter regimen, toxicity rate is considerably higher (38,39). Aggressive anthracycline-containing chemotherapy regimens should be reserved for patients with high tumor burden [bulky masses, unfavorable international prognostic index (IPI)] or for those with histologic transformation (40).
DLBCL of the stomach
Gastric DLBCLs are the most common lymphoma among primary GI lymphomas. Their clinical behavior as well as histology and prognostic factors are similar to their nodal counterpart. Half of them is associated with concomitant areas of MALT lymphoma (41). In general, the same guidelines followed for nodal DLCBL can be also applied to GI lymphomas with aggressive histology (42). In both, localized and advanced stage disease chemo-immunotherapy is recommended. The need of radiotherapy is controversial. A randomized clinical trial carried out by the IELSG between 1998 and 2004 tested the possible role of IF-RT as consolidation treatment after chemotherapy in gastric DLBCL. Fifty-four patients were enrolled and all received anthracycline containing regimens as induction. Patients were evaluated after four to six cycles and those in CR were randomized to receive IF-RT or two addition cycles of the same chemotherapy. Forty-five patients (83%) were randomized. The patients in the IF-RT arm showed a significant long-term reduction in the incidence of local relapse versus those who received chemotherapy alone. However, OS was not different between the two arms (43). This study suggests that irradiation improved the local control but, as it was performed prior to the introduction of rituximab, these results might no longer be valid in patients currently treated with immunochemotherapy.
Following the demonstration of MALT lymphoma regression after H. pylori eradication, this treatment approach was tested also in selected patients with early-stage H. pylori-positive DLBCL of the stomach (44). Subsequent studies showed that these patients can be managed with antibiotics alone, with high cure rates, keeping chemo-radiotherapy for unresponsive patients (45-47). A study from Taiwan, enrolling 50 patients with early stage DLBCL of the stomach, showed complete pathologic remissions in 69% of de novo gastric DLBCL patients and 56% of MALT-related DLBCL patients after anti-helicobacter therapy; at a median follow-up of approximately 8 years, all the responding patients were alive and free of lymphomas, except for one patient who died of lung cancer (46). A smaller Italian prospective trial confirmed that H. pylori eradication can result in long-term remission in patients with MALT-related DLBCL as well as in those with de novo gastric DLBCL, demonstrating that two-thirds of these patients can be efficiently managed with antibiotics alone, allowing a CR rate of 63%, a 5-year OS of 94%, with no deaths due to lymphoma (45). Before an antibiotic therapy alone can be considered ‘standard’ in the treatment of localized HP-positive gastric DLBCL, however, these results must be validated in prospective, wider-scale studies. A recent retrospective study compared two groups of patients with either H. pylori-positive or H. pylori-negative de novo gastric DLBCL (with no MALT component) uniformly treated by conventional chemotherapy. Interestingly, H. pylori-positive patients had lower IPI score, lower clinical stage, better tumor response to chemotherapy, and significantly superior 5-year EFS and OS (48). Whether H. pylori-positive de novo DLBCL may be a distinct, less aggressive and very chemosensitive tumor entity has yet to be elucidated.
Intestinal lymphomas
The most common localization for intestinal lymphoma is the small bowel, while large bowel or rectal lymphomas are less common. The majority of primary intestinal B-cell lymphomas are DLBCL, though other histologic subtypes can include intestinal MALT lymphoma [which often presents the features of the immunoproliferative small intestinal disease (IPSID)], MCL, or FL. Signs and symptoms at presentation are non-specific, varying from feeling of abdominal fullness and/or pain, nausea, diarrhea to bowel obstruction and perforation. These symptoms eventually lead to laparoscopy and bowel resection for the diagnosis. The outcome of intestinal lymphomas depends on the extent of the disease and on the histologic subtype. In a large series of intestinal lymphomas, an overall 5-year survival of 60-75% is reported for patients with B-cell lymphomas (49,50). Usually intestinal lymphomas are managed with surgery followed by immunochemotherapy (49,50). For patients with rectal disease, treatment usually includes immunochemotherapy and RT (30-40 Gy) in patients with DLBCL, while IF-RT alone (30-35 Gy) can provide long-term disease control in those with MALT lymphomas.
Immunoproliferative small intestinal disease (IPSID)
The term IPSID refers to the MALT lymphoma arising in the small bowel. This peculiar lymphoma has a long natural history, often over many years. In its early phase it is potentially reversible and can be treated with prolonged antibiotic therapy (i.e., tetracycline or metronidazole and ampicillin for at least six months), which can allow a lymphoma regression. This peculiar behavior has suggested a pathogenetic role for an infectious agent, Campylobacter jejuni seeming the best candidate (51). However, if left untreated, the lymphoma can undergo a histologic transformation to a DLBCL. Anthracycline containing regimens, together with nutritional support and antibiotics to control diarrhea and malabsorption, represent the best curative approach for advanced stage disease. Surgery has no therapeutic role, since this lymphoma is usually multifocal.
Multiple lymphomatous polyposis
This term refers to the peculiar clinical presentation of the GI-lymphoma as multiple lymphomatous polyps of the intestine. It is a heterogeneous group of lymphomas. In the vast majority of cases the underlying disease is a MCL; only rarely this clinical syndrome can be associated with other histologies (52). Therefore, the term multiple lymphomatous polyposis does not define a specific histopathological entity.
GI Burkitt’s lymphoma
Extranodal sites (most commonly bone, GI-tract, gonads, kidneys, thyroid, and breast) are usually involved in BL, with some differences according to the clinical variants. The majority of sporadic BL cases presents an abdominal mass, and the ileocecal region is the GI-site most frequently involved. The histologic and cytogenetic features are similar to the classical endemic (African) form, which most commonly affects the facial bone, but may also involve the distal ileum, cecum and omentum. Differently from BL arising in immunocompetent individuals, HIV-associated BL may present prominent nodal involvement; testis and bone marrow are also common sites. The most appropriate BL treatment strategy requires intensive combination chemotherapy. Surgery and RT as locoregional therapy do not provide adequate control of the disease. Nevertheless, surgery is important to establish the diagnosis and may be beneficial in case of abdominal emergencies.
Primary lymphoma of the testis
Primary NHL of the testis accounts for about 9% of testicular neoplasms and 1-2% of all NHL with an estimated incidence of 0.26/100,000 per year overall (53). NHL is the most common testicular malignancy in adult patients. The median age at diagnosis of the testis lymphoma is in the sixth decade. DLBCL is the most common histologic subtype seen in testis lymphoma (80-90% of the cases) (53), but isolated cases of other histologic subtypes have been described such as Burkitt’s and Burkitt’s-like types (10-20%), mainly in HIV-infected patients. FL involving the testis is very rare and has been reported mainly in childhood.
Orchiectomy is usually required for the pathological diagnosis; it also removes the potential chemotherapy sanctuary site generated by the blood-testis barrier.
The disease is limited to testis, stage IE, in most patients and approximately 20% have stage II disease. Disseminated disease is unusual (54), a stage IV testicular lymphoma being indeed virtually indistinguishable from a nodal one with testicular involvement. The most frequent clinical presentation is a unilateral painless scrotal swelling. Systemic B symptoms are usually present only in advanced stage. Moreover, patients can present abdominal pain, ascites, or hydrocele (43% of cases). Bilateral testicular involvement has been detected in up to 35% of patients. It may be synchronous at diagnosis or, more frequently, asynchronous during the course of the disease, with a 3- and 15-year risk of 15% and 40%, respectively (55,56), but this is virtually limited to patients who did not receive prophylactic scrotal radiotherapy (55,57). In addition to the contralateral testis, the disease typically spreads to other extranodal sites such as the skin and subcutaneous tissue, the lungs, the bone, and mainly the CNS (55). In the largest series of 373 retrospective patients with testis lymphoma, the IELSG observed a 5- and 10-year incidence of CNS relapse of 20% and 35%, respectively (55). The staging should therefore include cerebrospinal fluid (CSF) cytology. However, the pattern of CNS involvement typically displays a propensity for parenchymal involvement and for late relapses (55), which is clearly different from primary nodal DLBCL, where the CNS relapse rate is around 5% and relapses are typically early and leptomeningeal. Testis lymphomas have been considered very aggressive malignancies, with a poor outcome. In fact, in spite of initial CR, most patients with stage I-II disease relapse. The above mentioned large retrospective IELSG series reported a 5- and 10-year OS rate of 48% and 27%, respectively. The OS and PFS survival curves showed no clear evidence of plateau, suggesting no cure for these patients, even for those with stage I-II disease (55). Most of the patients in this study did not receive CNS prophylaxis, but in patients who received anthracycline-based chemotherapy plus intrathecal prophylaxis and scrotal irradiation the outcome was significantly better (5-year PFS, 72%) (55). This benefit led to the IELSG-10 phase II trial, which showed that combined treatment with six cycles of R-CHOP-21, intrathecal MTX, and contralateral testis irradiation was associated with a good outcome in stage I-IIE disease (5-year PFS and OS rates were 74% and 85%, respectively), and RT eliminated the contralateral testis relapses (57). Patients with disseminated disease should be treated according to the guidelines for the treatment of advanced stage nodal DLBCL, with the addition of prophylactic scrotal radiotherapy and intrathecal chemotherapy. Routine CNS prophylaxis is recommended in testis lymphoma patients of any stage since the high rate of CNS recurrence. However, despite most CNS recurrences are parenchymal, the benefit of intravenous high-dose methotrexate (HD-MTX) as CNS prophylaxis has never been formally demonstrated (58). This approach, which may be a sensible choice at least in the younger and fit patients, is being currently tested by the IELSG-30 trial (A phase II study of R-CHOP with systemic and intrathecal CNS prophylaxis and scrotal irradiation in patients with primary testicular DLBCL; NCT00945724).
Primary CNS lymphomas (PCNSLs)
PCNSL is a rare subtype of NHL that is characterized by the primary and exclusive involvement of the brain, spinal cord, leptomeninges and eyes. It represents 4% of intracranial cancers and 4-6% of primary extranodal lymphomas (59). Immunodeficiency is the only established risk factor for the development of PCNSL; patients with HIV infection have a 3,600-fold increased risk compared with the general population (60). In HIV-infected individuals EBV infection is often detected and, in addition to DLBCL, classic or atypical BL histology can be seen. The large majority of PCNSL in immunocompetent individuals are DLBCL. Indolent subtypes (usually small lymphocytic and lymphoplasmacytic lymphomas) are extremely rare. In the immunocompetent patient, the median age at onset is in the 60th decade and EBV infection is usually absent (60,61).
Patients usually present neurological symptoms based on the disease site: focal deficits, neuropsychiatric symptoms, seizures and manifestations of increased intracranial pressure. In 20% of the cases, a contemporary involvement of the eyes is reported (60). In patients with ocular involvement, the diagnosis can be made by stereotactic brain biopsy, CSF cytopathology, or analysis of vitreous aspirate. The International PCNSL Collaborative Group (IPCG) has indicated guidelines for diagnosis (62), standardized baseline evaluation of newly diagnosed PCNSL (63) as well as for neurocognitive assessment (64). A large retrospective study of the IELSG has developed a 5-point scoring system based on five prognostic variables: age older than 60 years, ECOG performance status higher than 1, elevated LDH level, high CSF total protein concentration, and involvement of deep regions of the brain. Patients with no or one risk factor had a 2-year OS of 80%, patients with four or five adverse risk factors had a 2-year OS of 15% (65). A different prognostic score, which allocates patients into three groups with significantly different OS and failure-free survival, has been proposed by the Memorial Sloan-Kettering Cancer Center based solely on age and Karnofsky performance status (66).
PCNSL is sensitive to both chemotherapy and radiotherapy, but the overall response rates and long-term survival appears inferior to the results achieved in similar subtypes of extranodal NHL. Yet, there are no standard guidelines for therapy of PCNSL. Since PCNSL is considered a disseminated and infiltrating disease, focal RT is not recommended and whole brain radiation therapy (WBRT) alone is rarely curative. Cranio-spinal irradiation does not add further benefit over WBRT, but increases toxicity. WBRT alone may have a role in the rare cases of PCNSL with indolent histology or for the palliative treatment of unfit patients who have contraindication to chemotherapy (59,67). PCNSL treatment is usually based on the use of HD-MTX at doses ranging between 1 to 8 g/m2 either as single agent or in combination with other chemotherapeutic agents and/or radiotherapy. There is no consensus on the dose of HD-MTX and the optimal combination regimen or on the role of radiation associated with methotrexate as first-line therapy. A randomized phase II study of the IELSG showed improved efficacy and activity with the addition of high-dose cytarabine (2 g/m2 twice a day on days 2-3) to HD-MTX (3.5 g/m2 on day 1) and demonstrated that combination chemotherapy in patients less than 75 years old is better than methotrexate alone (CR rate, 46% vs. 18%; 3-year OS, 46% and 32%, respectively) (68). Thus, HD-MTX plus cytarabine combination should be currently considered a standard regimen for PCNSL patients based on the best level of evidence available (69,70). The combination of HD-MTX based chemotherapy plus WBRT, despite a good 2-year OS rate (40-70%), has failed to improve long-term survival (5-year OS around 25%) (62), while the combination of high-dose MTX and cytarabine followed by WBRT allowed to obtain a 5-year OS rate of approximately 45% (69). However, the addition of WBRT may increase the risk of treatment-related neurotoxicity, particularly in the elderly. Thus, the omission of RT (at least in those who have achieved a CR with chemotherapy) has been proposed as a strategy to minimize the neuropsychological side effects. Nevertheless, particularly for patients unresponsive to chemotherapy, WBRT seems necessary, since no efficacious alternatives are available. In a phase III trial of the German PCNSL study group, patients were randomized to receive HD-MTX-based chemotherapy with or without WBRT. The authors concluded that, when WBRT is omitted from first-line chemotherapy, no significant difference in OS could be demonstrated. For this reason the PFS benefit afforded by WBRT should be weighed against the increased risk of long-term neurotoxicity (71). The interpretation of this trial has generated a still unsolved controversy and it seems difficult to draw reliable conclusions on the effect of WBRT on either survival or neurotoxicity (72,73). Though at present no definitive recommendations can be made on the consolidative role of WBRT after HD-MTX based regimens, it seems advisable at least in patients with residual disease. Lowering the WBRT dose may be an alternative approach (59): a prospective study showed no neurocognitive decline after consolidation with a reduced dose of 23.4 Gy in elderly patients who had achieved a CR after HD-MTX-based chemotherapy (74-76).
High-dose chemotherapy with autologous stem cell transplantation (HDC/SCT) has been proposed to improve CNS bioavailability of anticancer drugs and to replace WBRT with a less neurotoxic approach (77-79). The outcome after HDC/SCT seems related to the inclusion in the conditioning regimens of drugs (busulphan, thiotepa, BCNU) that efficiently cross the blood-brain barrier (59).
Two phase II trials exploring the use of HDC/SCT versus brain irradiation after induction chemotherapy in PCNSL patients are currently ongoing (NCT01011920, NCT00863460). Theoretically, HDC/SCT represents a possibility to replace WBRT, at least in younger subjects, in an effort to avoid treatment-related neurotoxicity. At present, however, its role remains investigational.
Primary lymphoma of the bone
Primary bone lymphomas (PBL) represent around 5% of all primary extranodal NHLs, and 3-7% of all bone cancers. At present, three forms of bone lymphoma can be described: the PBL consisting in a single bone lesion with or without regional lymphadenopathies, the polyostotic lymphoma characterized by a multifocal disease exclusively involving the skeleton, and the disseminated lymphoma with secondary infiltration of the bone. The former two groups should be considered on the whole as PBLs. The reported median age at the diagnosis varies from 45 to 60 years, with a slight male predominance. Patients present typically bone pain (80-95%), tumor mass is present in 30-40% of the cases with pathological fracture in 10-15% of them (80,81). Spinal cord compression is present in 14% of the patients. Hypercalcemia and osteolysis are described in 10% and 15% of the cases, respectively, mainly in the presence of progressive disease. The time between the disease’s onset and the diagnosis is generally short (8 months). Most patients have an early-stage disease (82) and the femur is the commonest reported site of involvement. However, some Japanese reports have indicated the pelvic bones as the most common site of disease (83), suggesting that a geographical variability may be present. Small bones of hands and fingers are rarely involved. DLBCL is the most common lymphoma type involving primarily or secondarily the bone, accounting for 70-80% of bone lymphomas (83,84). The occurrence of FL, MZL, LPL, anaplastic large cell lymphoma, BL and Hodgkin’s lymphoma has also been reported (85).
The diagnosis requires a surgical biopsy to define the histologic type of lymphoma. Radiographic findings are usually non-specific and the differential diagnosis between lymphoma and solid cancer is often difficult. While computed tomography (CT) scan is the main method of staging, restaging and follow-up of PBL as well as nodal lymphomas (86), magnetic resonance imaging (MRI) better detects the local extent of the disease and cortical changes (87,88). PET-CT scan has become the standard imaging technique for staging of PBL, despite the fact that data derive from small retrospective series (89-91). Staging should be performed using the Ann Arbor staging system. Yet, some limitations of this system exist, and an adapted Ann Arbor staging system for PBL has been proposed (Table 2).

Full table
The prognosis of primary bone DLBCL depends mainly on the stage: 5-year OS vary from 82% for stage IE disease to 38% for disseminated DLBCL with bone localizations. Local and systemic relapses for patients in early stage occur in 10% and 17% of the cases, respectively (92). Polyostotic lymphoma relapses, which usually involve the skeleton with systemic sites in 21% of the cases, are associated with a bad outcome (80). The role of the IPI in predicting prognosis seems limited. The IELSG-14 study identified five prognostic factors associated with a better outcome: age <60 years, normal LDH levels, combined modality therapy, higher RT dose, ECOG score 0-2 (80,81,85,93). The treatment approach differs on the basis of the histologic type and disease stage. Stage I-IIE primary bone DLBCL should be treated with anthracycline-containing chemotherapy regimens with or without following IF-RT (81). This strategy allows to obtain >90% ORR and a 5-year OS of 84%. The benefit of the addition of rituximab has not been properly studied, and most data on the treatment of primary bone DLBCL come from the pre-rituximab era. However, while it is reasonable to expect a benefit from the addition of rituximab to CHOP in the case of nodal DLBCL (94-96), in a retrospective survey of the German High-Grade Non-Hodgkin Lymphoma Study Group rituximab failed to improve the outcome of patients with DLBCL with skeletal involvement. On the contrary, there was an apparent beneficial effect of radiotherapy to sites of skeletal involvement (97). The impact in terms of outcome of the consolidative RT after chemotherapy is indeed controversial (81,97) and requires further validation in prospective trials. The large retrospective IELSG-14 study showed no benefit in terms of PFS when whole-bone irradiation is given and no impact of different RT doses (81).
In conclusion, chemo-immunotherapy followed or not by RT remains the standard approach for the patients with advanced stage DLBCL with bone involvement. Polyostotic lymphomas should be treated the same as disseminated DLBCL, considering RT when feasible (80). Given their rarity (5%), there are only few data on the management of indolent bone lymphomas. Globally, patients with localized disease are candidate for RT alone (85,98), while patients with disseminated disease can be managed the same as patients with other disseminated indolent lymphomas.
Head and neck lymphomas
Primary lymphoma of the Waldeyer’s ring
Despite arising in a lymphatic site, Waldeyer’s ring lymphomas are included by many authors among the extranodal lymphomas (2,99). Tonsils are the commonest site of involvement in Waldeyer’s ring, other sites including the nasopharynx and the base of the tongue. DLBCL is the most frequent histologic type, but a wide spectrum of histologies comprising FL, MALT, and other small cell lymphomas has been described. Clinical presentation includes dysphagia, airway obstruction, or a mass lesion in the throat, with a frequent involvement of the GI tract at onset, which should be verified at staging. A combined treatment with chemotherapy and RT is considered a valid therapeutic approach in patients with localized NHL, either nodal or extranodal, in most anatomic sites, including Waldeyer’s ring (100-102). However, the combinatory treatment is often associated with relevant acute and chronic toxicity (99,103). In a large retrospective series of the IELSG, consolidation radiotherapy did not prolong lymphoma-specific survival in patients with early-stage DLBCL of Waldeyer’s ring who achieved a CR after anthracycline-containing chemotherapy (104). This seems to suggest that RT could be omitted in patients achieving CR, sparing avoidable toxicity that may affect patients’ quality of life (99,104). This finding needs to be further confirmed in prospective studies.
Primary lymphoma of the thyroid
Primary thyroid lymphomas are uncommon, accounting for only 1-5% of thyroid neoplasms and less than 2% of extranodal lymphomas, and usually affects elderly women (2). In the large majority of cases they are MALT lymphomas and DLBCL with or without a MALT component. The development of a primary thyroid MALT lymphoma can be considered a late event related to the acquisition of intra-thyroid lymphoid tissue in the context of an autoimmune thyroid disease such as Hashimoto’s thyroiditis (99,105,106). Frequently patients present an enlarging neck mass; more rarely, this is accompanied by compression of surrounding structures and related symptoms such as dysphagia and/or dyspnea. In the large majority of patients the disease is localized. In case of disease dissemination, the involvement of the GI tract is frequent, while CNS involvement is rare.
Despite some historical series have reported an excellent local control in localized primary thyroid MALT lymphomas treated only with radical thyroidectomy, surgery can be important in establishing the diagnosis. Surgery can’t however be considered curative, but may be beneficial in case of emergencies such as airway obstruction.
Patients with localized MALT lymphomas have excellent clinical outcome after moderate-dose RT (30 Gy) (29). Thyroid (and gastric) MALT lymphomas are found to have significantly less risk of distant recurrence, and, differently from other histologic subtypes, may not require a combined modality approach (35). The standard treatment approach for disseminated indolent disease and for all stages of primary thyroid DLBCL should be the combination of chemotherapy and radiotherapy (107). In a series of 48 poor-risk primary thyroid DLBCL, combined treatment modality has been shown to induce an elevated rate of CR and to improve long-term survival in younger patients (108).
Primary lymphoma of the salivary gland
Salivary gland lymphomas range between 5% to 10% of all salivary gland cancers and less than 5% of all lymphomas. They usually originate in the parotid gland. The more frequent histologic subtypes are MALT lymphoma and DLBCL either de novo or arising from a preceding MZL. In a retrospective series of 180 patients analyzed by the IELSG group, the salivary gland was the commonest site among non-gastric MALT lymphomas (109). Similarly to other MALT lymphomas, there is some evidence of a correlation between the lymphoma development and chronic antigenic stimulation associated either with Sjogren’s syndrome or chronic hepatitis C virus infection (109,110). Clinical presentation is characterized by the presence of a painless mass in the parotid or submandibular gland, frequently bilateral. The role of surgery is limited to excisional biopsy required for the diagnosis and is not recommended as curative option. In localized primary salivary gland MALT lymphoma, an excellent disease control is achieved with RT alone. Immunochemotherapy is recommended for patients with advanced stage disease or in cases with pre-existing xerostomia related to the Sjogren’s syndrome (111). Anthracycline-containing chemotherapy regimens with rituximab or combined modality treatments are indicated for primary salivary gland DLBCL.
Primary ocular adnexa lymphoma
Orbital lymphomas comprise those lymphomas primarily involving the ocular adnexa, such as the conjunctiva, eyelids, lacrimal glands, or retro-orbital soft tissues. This lymphoma subtype is more frequent than the intraocular lymphoma (or lymphoma of the eye), which is a PCNSL and has a different clinical course. The lymphomas of ocular adnexa usually present anterior lesions, small nodules in the conjunctiva, and symptoms of blurred vision. In the presence of posterior lesions, patients present swelling and/or proptosis. Pain is rare, but pressure and diplopia may occur depending on the lesions’ size. These lymphomas can have different histological types and treatment should be driven by the pathological diagnosis. Nevertheless, histology is usually extranodal marginal zone B-cell lymphoma of MALT type (112), and it is worthwhile to describe separately this subtype because its development has been linked with chronic Chlamydophila psittaci (C. psittaci) infection (113).
Primary ocular adnexa marginal zone lymphomas of MALT type (OAMZL)
The development of ocular adnexa MALT lymphoma is frequently associated with chronic conjunctivitis. These lymphomas have an excellent prognosis, irrespective of initial therapy, as there doesn’t seem to be any significant difference in time-to-progression and response between local or systemic therapy, suggesting that the less toxic individual approach should be chosen (114). In an Italian study, C. psittaci was detected in up to 80% of patients with ocular adnexa lymphoma. This finding provided the rationale for the antibiotic treatment of localized lesions (112,113). Moreover, a pivotal Italian experience showed that the eradication of C. psittaci infection using doxycycline for patients with OAMZL may result in lymphoma regression in approximately 50% of patients, including pre-treated patients and patients with regional lymph node involvement (115,116). Following this first demonstration, a prospective international phase II study was later conducted by the IELSG. Thirty-four patients were treated front-line with doxycycline and a lymphoma regression was observed in 22 patients (65%; 95% CI, 49-81%), with 16 partial responses and six complete responses. At a median follow-up of 37 months, the 5-year PFS was 55% (117). Globally, doxycycline has been tested in 120 patients with OAMZL, allowing an overall response rate of approximately 50% (118). Despite these promising results, it is necessary to further improve the efficacy of antibiotics either by clarifying the optimum schedule of doxycycline or by identifying other possible infectious agents and the appropriate time to start a lymphoma-treatment when eradication fails (117,119,120).
Primary lymphoma of the breast
Primary breast lymphoma accounts for around 2% of localized extranodal NHL presentations and is defined by the presence of a primary lesion within the breast, with or without regional nodal involvement and with no other extra-mammary sites (121). DLBCL is the most common histologic subtype. However, FL, MALT lymphomas and BL have also been described (122,123). A retrospective international series of 204 primary breast DLBCL published by the IELSG described some peculiar features of this clinical entity compared to nodal DLBCL, namely, a significant risk of contralateral breast and other extranodal sites relapse and a high rate of CNS relapse. This study showed that the IPI retained its significant prognostic value for OS and PFS as in nodal DLBCL (122). Except for a lower incidence of CNS relapse reported in the IELSG survey, these data were in line with previous published data on the same topic (124-126). In the IELSG patient population receiving chemotherapy without immunotherapy in 80% of the cases, mainly with anthracycline-containing regimens or chemotherapy combined with radiotherapy, the median OS was 8 years, with 5- and 10-year OS rates of 63% and 47% respectively; median PFS was 5.5 years (122). Based on these data, the combination of anthracycline-containing chemotherapy and loco-regional radiotherapy seems adequate, following a diagnostic surgical biopsy. Although the addition of rituximab has never been studied, it is likely to be beneficial.
Less information is available on the rare indolent lymphomas primarily arising in the breast. In a retrospective international survey of 60 cases with histological revision (36 FL and 24 MZL), surgery, chemotherapy and radiotherapy, alone or in combination, were used as first-line treatments in 67%, 42% and 52% of patients, respectively. The 5-year PFS was 56% for MZL and 49% for FL and relapses were mostly in distant sites (18 of 23 cases, with no patients relapsing within radiotherapy fields) (123).
Primary B-cell lymphoma of the skin
The WHO 2008 classification of Tumors of Hematopoietic and Lymphoid Tissues (127) recognizes three main types of cutaneous lymphomas, namely the extranodal MZL of MALT (PCMZL), the primary cutaneous follicle center lymphoma (PCFCL), and the primary cutaneous DLBCL, leg-type (PCLBCL-LT). The indolent primary cutaneous B-cell lymphomas subtypes (PCMZL and PCFCL) generally have a good prognosis, but they often relapse leading in some cases to extra-cutaneous disease spread and, therefore, to poorer survival. A retrospective study of the IELSG on 270 patients affected by indolent primary cutaneous B-cell lymphomas identified high levels of serum LDH, the presence of nodular skin lesions and the presence of more than two skin lesions as main predictors of PFS (128).
PCMZLs comprise about 7% of all cutaneous lymphomas (129). The commonest presentations (one or more papules, nodules, or plaques) are located on the extremities or the trunk. They are usually treated with radiotherapy (or surgical excision alone) and rarely spread to extra-cutaneous sites (127,130).
PCFCL account for about 10% of cutaneous lymphomas (129). Clinical presentation is characterized by erythematous papules and solitary or grouped plaques, sometimes associated with nodules as well (127). Cutaneous lesions are usually found on the head and neck or trunk regions (rarely on legs). Some cases can progress to a DLBCL or a coexistent DLBCL can be found (131).
PCLBCL-LT is an aggressive lymphoma occurring most frequently in elderly women (130). It usually, but not always, appears on the (lower) leg. The dissemination of this type of lymphoma to extra-cutaneous sites is higher and is reported in 50% of the cases (130). PCMZL and PCFCL are indolent types of CBCL with a disease-related 10-year survival rate of 90% (130,132,133), while PCLBCL-LT has a more unfavorable prognosis (disease-related 5-year survival of 50%) (130).
The EORTC/ISCL consensus recommendations for the management of these three types of CBCL are summarized in Table 3 (134,135). Curative radiation doses recommended for localized PCMZL and PCFCL are 24-36 Gy, while palliative treatment of multifocal disease may be based on low-dose radiation (4 Gy). Higher radiation dose (40 Gy) is recommended for PCLBCL-LT only (135).
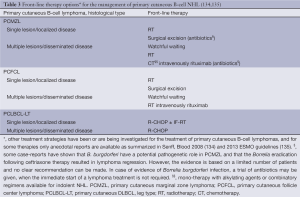
Full table
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Zucca E, Roggero E, Bertoni F, et al. Primary extranodal non-Hodgkin's lymphomas. Part 1: Gastrointestinal, cutaneous and genitourinary lymphomas. Ann Oncol 1997;8:727-37. [PubMed]
- Zucca E, Roggero E, Bertoni F, et al. Primary extranodal non-Hodgkin's lymphomas. Part 2: Head and neck, central nervous system and other less common sites. Ann Oncol 1999;10:1023-33. [PubMed]
- Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg 1961;49:80-9. [PubMed]
- Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract: a study of 117 cases presenting with gastrointestinal disease. Cancer 1978;42:693-707. [PubMed]
- Herrmann R, Panahon AM, Barcos MP, et al. Gastrointestinal involvement in non-Hodgkin's lymphoma. Cancer 1980;46:215-22. [PubMed]
- d’Amore F, Christensen BE, Brincker H, et al. Clinicopathological features and prognostic factors in extranodal non-Hodgkin lymphomas. Danish LYFO Study Group. Eur J Cancer 1991;27:1201-8. [PubMed]
- Groves FD, Linet MS, Travis LB, et al. Cancer surveillance series: non-Hodgkin’s lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst 2000;92:1240-51. [PubMed]
- Müller AM, Ihorst G, Mertelsmann R, et al. Epidemiology of non-Hodgkin’s lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol 2005;84:1-12. [PubMed]
- Devesa SS, Fears T. Non-Hodgkin’s lymphoma time trends: United States and international data. Cancer Res 1992;52:5432s-40s. [PubMed]
- Otter R, Gerrits WB. Primary extranodal and nodal non-Hodgkin’s lymphoma. A survey of a population-based registry. Eur J Cancer Clin Oncol 1989;25:1203-10. [PubMed]
- Otter R, Willemze R. Extranodal non-Hodgkin’s lymphoma. Neth J Med 1988;33:49-51. [PubMed]
- Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer 1972;29:252-60. [PubMed]
- d’Amore F, Brincker H, Grønbaek K, et al. Non-Hodgkin’s lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol 1994;12:1673-84. [PubMed]
- Koch P, del Valle F, Berdel WE, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001;19:3861-73. [PubMed]
- Shenkier TN, Connors JM. Primary extranodal non-Hodgkin’s lymphomas. In: Canellos GP, Lister TA, Young BD. eds. The Lymphomas, 2nd edition. Philadelphia, PA: Saunders Elsevier, 2006:325-47.
- Zucca E, Cavalli F. Gut lymphomas. Baillieres Clin Haematol 1996;9:727-41. [PubMed]
- Bertoni F, Coiffier B, Salles G, et al. MALT lymphomas: pathogenesis can drive treatment. Oncology (Williston Park) 2011;25:1134-42. [PubMed]
- Bertoni F, Zucca E. State-of-the-art therapeutics: marginal-zone lymphoma. J Clin Oncol 2005;23:6415-20. [PubMed]
- Krol AD, Hermans J, Kramer MH, et al. Gastric lymphomas compared with lymph node lymphomas in a population-based registry differ in stage distribution and dissemination patterns but not in patient survival. Cancer 1997;79:390-7. [PubMed]
- Zucca E, Copie-Bergman C, Ricardi U, et al. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi144-8. [PubMed]
- Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut 2011;60:747-58. [PubMed]
- Copie-Bergman C, Wotherspoon AC, Capella C, et al. Gela histological scoring system for post-treatment biopsies of patients with gastric MALT lymphoma is feasible and reliable in routine practice. Br J Haematol 2013;160:47-52. [PubMed]
- Capelle LG, de Vries AC, Looman CW, et al. Gastric MALT lymphoma: epidemiology and high adenocarcinoma risk in a nation-wide study. Eur J Cancer 2008;44:2470-6. [PubMed]
- Wündisch T, Dieckhoff P, Greene B, et al. Second cancers and residual disease in patients treated for gastric mucosa-associated lymphoid tissue lymphoma by Helicobacter pylori eradication and followed for 10 years. Gastroenterology 2012;143:936-42; quiz e13-4.
- Thieblemont C, Dumontet C, Bouafia F, et al. Outcome in relation to treatment modalities in 48 patients with localized gastric MALT lymphoma: a retrospective study of patients treated during 1976-2001. Leuk Lymphoma 2003;44:257-62. [PubMed]
- Pinotti G, Zucca E, Roggero E, et al. Clinical features, treatment and outcome in a series of 93 patients with low-grade gastric MALT lymphoma. Leuk Lymphoma 1997;26:527-37. [PubMed]
- Yahalom J. Patients with H pylori-independent MALT lymphoma are curable with radiotherapy. Oncology (Williston Park) 2011;25:1147-9. [PubMed]
- Wirth A, Gospodarowicz M, Aleman BM, et al. Long-term outcome for gastric marginal zone lymphoma treated with radiotherapy: a retrospective, multi-centre, International Extranodal Lymphoma Study Group study. Ann Oncol 2013;24:1344-51. [PubMed]
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003;21:4157-64. [PubMed]
- Hitchcock S, Ng AK, Fisher DC, et al. Treatment outcome of mucosa-associated lymphoid tissue/marginal zone non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys 2002;52:1058-66. [PubMed]
- Milgrom SA, Yahalom J. Indolent non-Hodgkin lymphoma primarily involving the hard palate: outcome following radiotherapy. Leuk Lymphoma 2013;54:1208-11. [PubMed]
- NCCN. National Comprehensive Cancer Network Guidelines V.2.2013 - Non-Hodgkin’s Lymphoma. Available online: http://www.nccn.org/professionals/physician_gls/PDF/nhl.pdf. Accessed on 11/11/2013.
- Yahalom J. MALT lymphomas: a radiation oncology viewpoint. Ann Hematol 2001;80 Suppl 3:B100-5. [PubMed]
- Tsang RW, Gospodarowicz MK. Radiation therapy for localized low-grade non-Hodgkin’s lymphomas. Hematol Oncol 2005;23:10-7. [PubMed]
- Goda JS, Gospodarowicz M, Pintilie M, et al. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer 2010;116:3815-24. [PubMed]
- Zucca E, Conconi A, Laszlo D, et al. Addition of rituximab to chlorambucil produces superior event-free survival in the treatment of patients with extranodal marginal-zone B-cell lymphoma: 5-year analysis of the IELSG-19 Randomized Study. J Clin Oncol 2013;31:565-72. [PubMed]
- Zucca E, Conconi A, Martinelli G, et al. Chlorambucil plus rituximab produces better event-free and progression-free survival in comparison with chlorambucil or rituximab alone in extranodal marginal zone B-cell lymphoma (MALT lymphoma): results of the IELSG-19 study. Hematol Oncol 2013;31:97-8.
- Salar A, Domingo-Domenech E, Panizo C, et al. Bendamustine plus rituximab in first line systemic treatment for extranodal MALT lymphoma: final results of phase II trial of the Spanish lymphoma study group (GELTAMO). Hematol Oncol 2013;31:129-30.
- Brown JR, Friedberg JW, Feng Y, et al. A phase 2 study of concurrent fludarabine and rituximab for the treatment of marginal zone lymphomas. Br J Haematol 2009;145:741-8. [PubMed]
- Thieblemont C. Clinical presentation and management of marginal zone lymphomas. Hematology Am Soc Hematol Educ Program 2005;307-13. [PubMed]
- Ferreri AJ, Freschi M, Dell’Oro S, et al. Prognostic significance of the histopathologic recognition of low- and high-grade components in stage I-II B-cell gastric lymphomas. Am J Surg Pathol 2001;25:95-102. [PubMed]
- Ghielmini M, Vitolo U, Kimby E, et al. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL). Ann Oncol 2013;24:561-76. [PubMed]
- Martinelli G, Gigli F, Calabrese L, et al. Early stage gastric diffuse large B-cell lymphomas: results of a randomized trial comparing chemotherapy alone versus chemotherapy + involved field radiotherapy. (IELSG 4). Leuk Lymphoma 2009;50:925-31. [PubMed]
- Chen LT, Lin JT, Shyu RY, et al. Prospective study of Helicobacter pylori eradication therapy in stage I(E) high-grade mucosa-associated lymphoid tissue lymphoma of the stomach. J Clin Oncol 2001;19:4245-51. [PubMed]
- Ferreri AJ, Govi S, Raderer M, et al. Helicobacter pylori eradication as exclusive treatment for limited-stage gastric diffuse large B-cell lymphoma: results of a multicenter phase 2 trial. Blood 2012;120:3858-60. [PubMed]
- Kuo SH, Yeh KH, Wu MS, et al. Helicobacter pylori eradication therapy is effective in the treatment of early-stage H pylori-positive gastric diffuse large B-cell lymphomas. Blood 2012;119:4838-44. [PubMed]
- Cuccurullo R, Govi S, Ferreri AJ. De-escalating therapy in gastric aggressive lymphoma. World J Gastroenterol 2014;20:8993-7. [PubMed]
- Kuo SH, Yeh KH, Chen LT, et al. Helicobacter pylori-related diffuse large B-cell lymphoma of the stomach: a distinct entity with lower aggressiveness and higher chemosensitivity. Blood Cancer J 2014;4:e220. [PubMed]
- Cortelazzo S, Rossi A, Oldani E, et al. The modified International Prognostic Index can predict the outcome of localized primary intestinal lymphoma of both extranodal marginal zone B-cell and diffuse large B-cell histologies. Br J Haematol 2002;118:218-28. [PubMed]
- Ibrahim EM, Ezzat AA, El-Weshi AN, et al. Primary intestinal diffuse large B-cell non-Hodgkin’s lymphoma: clinical features, management, and prognosis of 66 patients. Ann Oncol 2001;12:53-8. [PubMed]
- Lecuit M, Abachin E, Martin A, et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med 2004;350:239-48. [PubMed]
- Hashimoto Y, Nakamura N, Kuze T, et al. Multiple lymphomatous polyposis of the gastrointestinal tract is a heterogenous group that includes mantle cell lymphoma and follicular lymphoma: analysis of somatic mutation of immunoglobulin heavy chain gene variable region. Hum Pathol 1999;30:581-7. [PubMed]
- Vitolo U, Ferreri AJ, Zucca E. Primary testicular lymphoma. Crit Rev Oncol Hematol 2008;65:183-9. [PubMed]
- Magrath IT. eds. The lymphoid neoplasms, third ed. Boca Raton: CRC Press, 2010.
- Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol 2003;21:20-7. [PubMed]
- Shahab N, Doll DC. Testicular lymphoma. Semin Oncol 1999;26:259-69. [PubMed]
- Vitolo U, Chiappella A, Ferreri AJ, et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol 2011;29:2766-72. [PubMed]
- Seymour JF, Solomon B, Wolf MM, et al. Primary large-cell non-Hodgkin’s lymphoma of the testis: a retrospective analysis of patterns of failure and prognostic factors. Clin Lymphoma 2001;2:109-15. [PubMed]
- Ferreri AJ. How I treat primary CNS lymphoma. Blood 2011;118:510-22. [PubMed]
- Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol 2006;24:1281-8. [PubMed]
- Rubenstein JL, Gupta NK, Mannis GN, et al. How I treat CNS lymphomas. Blood 2013;122:2318-30. [PubMed]
- Ferreri AJ, Abrey LE, Blay JY, et al. Summary statement on primary central nervous system lymphomas from the Eighth International Conference on Malignant Lymphoma, Lugano, Switzerland, June 12 to 15, 2002. J Clin Oncol 2003;21:2407-14. [PubMed]
- Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23:5034-43. [PubMed]
- Correa DD, Maron L, Harder H, et al. Cognitive functions in primary central nervous system lymphoma: literature review and assessment guidelines. Ann Oncol 2007;18:1145-51. [PubMed]
- Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol 2003;21:266-72. [PubMed]
- Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 2006;24:5711-5. [PubMed]
- Nayak L, Batchelor TT. Recent advances in treatment of primary central nervous system lymphoma. Curr Treat Options Oncol 2013;14:539-52. [PubMed]
- Ferreri AJ, Reni M, Foppoli M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 2009;374:1512-20. [PubMed]
- Ferreri AJ, Licata G, Foppoli M, et al. Clinical relevance of the dose of cytarabine in the upfront treatment of primary CNS lymphomas with methotrexate-cytarabine combination. Oncologist 2011;16:336-41. [PubMed]
- Ansell SM, Rajkumar SV. Hematology: Trials and tribulations in primary CNS lymphoma. Nat Rev Clin Oncol 2010;7:125-6. [PubMed]
- Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol 2010;11:1036-47. [PubMed]
- Cabanillas F. How important is whole brain radiotherapy for treatment of primary CNS lymphoma? Lancet Oncol 2010;11:1011-2. [PubMed]
- Ferreri AJ, DeAngelis L, Illerhaus G, et al. Whole-brain radiotherapy in primary CNS lymphoma. Lancet Oncol 2011;12:118-9; author reply 119-20. [PubMed]
- Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol 2007;25:4730-5. [PubMed]
- Correa DD, Rocco-Donovan M, DeAngelis LM, et al. Prospective cognitive follow-up in primary CNS lymphoma patients treated with chemotherapy and reduced-dose radiotherapy. J Neurooncol 2009;91:315-21. [PubMed]
- Ferreri AJ, Verona C, Politi LS, et al. Consolidation radiotherapy in primary central nervous system lymphomas: impact on outcome of different fields and doses in patients in complete remission after upfront chemotherapy. Int J Radiat Oncol Biol Phys 2011;80:169-75. [PubMed]
- Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol 2008;26:2512-8. [PubMed]
- Illerhaus G, Marks R, Ihorst G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol 2006;24:3865-70. [PubMed]
- Illerhaus G, Müller F, Feuerhake F, et al. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica 2008;93:147-8. [PubMed]
- Messina C, Ferreri AJ, Govi S, et al. Clinical features, management and prognosis of multifocal primary bone lymphoma: a retrospective study of the international extranodal lymphoma study group (the IELSG 14 study). Br J Haematol 2014;164:834-40. [PubMed]
- Bruno Ventre M, Ferreri AJ, Gospodarowicz M, et al. Clinical features, management, and prognosis of an international series of 161 patients with limited-stage diffuse large B-cell lymphoma of the bone (the IELSG-14 study). Oncologist 2014;19:291-8. [PubMed]
- Horsman JM, Thomas J, Hough R, et al. Primary bone lymphoma: a retrospective analysis. Int J Oncol 2006;28:1571-5. [PubMed]
- Maruyama D, Watanabe T, Beppu Y, et al. Primary bone lymphoma: a new and detailed characterization of 28 patients in a single-institution study. Jpn J Clin Oncol 2007;37:216-23. [PubMed]
- Beal K, Allen L, Yahalom J. Primary bone lymphoma: treatment results and prognostic factors with long-term follow-up of 82 patients. Cancer 2006;106:2652-6. [PubMed]
- Govi S, Christie D, Mappa S, et al. The clinical features, management and prognosis of primary and secondary indolent lymphoma of the bone: a retrospective study of the International Extranodal Lymphoma Study Group (IELSG #14 study). Leuk Lymphoma 2014;55:1796-9. [PubMed]
- Phillips WC, Kattapuram SV, Doseretz DE, et al. Primary lymphoma of bone: relationship of radiographic appearance and prognosis. Radiology 1982;144:285-90. [PubMed]
- Stiglbauer R, Augustin I, Kramer J, et al. MRI in the diagnosis of primary lymphoma of bone: correlation with histopathology. J Comput Assist Tomogr 1992;16:248-53. [PubMed]
- Hicks DG, Gokan T, O’Keefe RJ, et al. Primary lymphoma of bone. Correlation of magnetic resonance imaging features with cytokine production by tumor cells. Cancer 1995;75:973-80. [PubMed]
- Paes FM, Kalkanis DG, Sideras PA, et al. FDG PET/CT of extranodal involvement in non-Hodgkin lymphoma and Hodgkin disease. Radiographics 2010;30:269-91. [PubMed]
- Juweid ME. FDG-PET/CT in lymphoma. Methods Mol Biol 2011;727:1-19. [PubMed]
- Seam P, Juweid ME, Cheson BD. The role of FDG-PET scans in patients with lymphoma. Blood 2007;110:3507-16. [PubMed]
- Cai L, Stauder MC, Zhang YJ, et al. Early-stage primary bone lymphoma: a retrospective, multicenter Rare Cancer Network (RCN) Study. Int J Radiat Oncol Biol Phys 2012;83:284-91. [PubMed]
- Govi S, Christie D, Messina C, et al. The clinical features, management and prognostic effects of pathological fractures in a multicenter series of 373 patients with diffuse large B-cell lymphoma of the bone. Ann Oncol 2014;25:176-81. [PubMed]
- Catlett JP, Williams SA, O’Connor SC, et al. Primary lymphoma of bone: an institutional experience. Leuk Lymphoma 2008;49:2125-32. [PubMed]
- Ramadan KM, Shenkier T, Sehn LH, et al. A clinicopathological retrospective study of 131 patients with primary bone lymphoma: a population-based study of successively treated cohorts from the British Columbia Cancer Agency. Ann Oncol 2007;18:129-35. [PubMed]
- Alencar A, Pitcher D, Byrne G, et al. Primary bone lymphoma--the University of Miami experience. Leuk Lymphoma 2010;51:39-49. [PubMed]
- Held G, Zeynalova S, Murawski N, et al. Impact of rituximab and radiotherapy on outcome of patients with aggressive B-cell lymphoma and skeletal involvement. J Clin Oncol 2013;31:4115-22. [PubMed]
- Reddy N, Greer JP. Primary bone lymphoma: a set of unique problems in management. Leuk Lymphoma 2010;51:1-2. [PubMed]
- Mian M, Capello D, Ventre MB, et al. Early-stage diffuse large B cell lymphoma of the head and neck: clinico-biological characterization and 18 year follow-up of 488 patients (IELSG 23 study). Ann Hematol 2013. [Epub ahead of print]. [PubMed]
- Avilés A, Delgado S, Ruiz H, et al. Treatment of non-Hodgkin’s lymphoma of Waldeyer’s ring: radiotherapy versus chemotherapy versus combined therapy. Eur J Cancer B Oral Oncol 1996;32B:19-23. [PubMed]
- Seymour JF, Pro B, Fuller LM, et al. Long-term follow-up of a prospective study of combined modality therapy for stage I-II indolent non-Hodgkin’s lymphoma. J Clin Oncol 2003;21:2115-22. [PubMed]
- Chang DT, Mendenhall NP, Lynch JW, et al. Long-term outcomes for stage I-II aggressive non-Hodgkin lymphoma of Waldeyer’s ring. Am J Clin Oncol 2009;32:233-7. [PubMed]
- Chang DT, Amdur RJ, Pacholke H, et al. Xerostomia in long-term survivors of aggressive non-Hodgkin’s lymphoma of Waldeyer’s ring: a potential role for parotid-sparing techniques? Am J Clin Oncol 2009;32:145-9. [PubMed]
- Mian M, Ferreri AJ, Rossi A, et al. Role of radiotherapy in patients with early-stage diffuse large B-cell lymphoma of Waldeyer’s ring in remission after anthracycline-containing chemotherapy. Leuk Lymphoma 2013;54:62-8. [PubMed]
- Thieblemont C, Mayer A, Dumontet C, et al. Primary thyroid lymphoma is a heterogeneous disease. J Clin Endocrinol Metab 2002;87:105-11. [PubMed]
- Derringer GA, Thompson LD, Frommelt RA, et al. Malignant lymphoma of the thyroid gland: a clinicopathologic study of 108 cases. Am J Surg Pathol 2000;24:623-39. [PubMed]
- Stein SA, Wartofsky L. Primary thyroid lymphoma: a clinical review. J Clin Endocrinol Metab 2013;98:3131-8. [PubMed]
- Mian M, Gaidano G, Conconi A, et al. High response rate and improvement of long-term survival with combined treatment modalities in patients with poor-risk primary thyroid diffuse large B-cell lymphoma: an International Extranodal Lymphoma Study Group and Intergruppo Italiano Linfomi study. Leuk Lymphoma 2011;52:823-32. [PubMed]
- Zucca E, Conconi A, Pedrinis E, et al. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood 2003;101:2489-95. [PubMed]
- Arcaini L, Burcheri S, Rossi A, et al. Prevalence of HCV infection in nongastric marginal zone B-cell lymphoma of MALT. Ann Oncol 2007;18:346-50. [PubMed]
- Conconi A, Martinelli G, Thiéblemont C, et al. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood 2003;102:2741-5. [PubMed]
- Ferreri AJ, Dolcetti R, Du MQ, et al. Ocular adnexal MALT lymphoma: an intriguing model for antigen-driven lymphomagenesis and microbial-targeted therapy. Ann Oncol 2008;19:835-46. [PubMed]
- Ferreri AJ, Guidoboni M, Ponzoni M, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst 2004;96:586-94. [PubMed]
- Wöhrer S, Kiesewetter B, Fischbach J, et al. Retrospective comparison of the effectiveness of various treatment modalities of extragastric MALT lymphoma: a single-center analysis. Ann Hematol 2014;93:1287-95. [PubMed]
- Ferreri AJ, Ponzoni M, Guidoboni M, et al. Regression of ocular adnexal lymphoma after Chlamydia psittaci-eradicating antibiotic therapy. J Clin Oncol 2005;23:5067-73. [PubMed]
- Ferreri AJ, Ponzoni M, Guidoboni M, et al. Bacteria-eradicating therapy with doxycycline in ocular adnexal MALT lymphoma: a multicenter prospective trial. J Natl Cancer Inst 2006;98:1375-82. [PubMed]
- Ferreri AJ, Govi S, Pasini E, et al. Chlamydophila psittaci eradication with doxycycline as first-line targeted therapy for ocular adnexae lymphoma: final results of an international phase II trial. J Clin Oncol 2012;30:2988-94. [PubMed]
- Kiesewetter B, Raderer M. Antibiotic therapy in nongastrointestinal MALT lymphoma: a review of the literature. Blood 2013;122:1350-7. [PubMed]
- Kim TM, Kim KH, Lee MJ, et al. First-line therapy with doxycycline in ocular adnexal mucosa-associated lymphoid tissue lymphoma: a retrospective analysis of clinical predictors. Cancer Sci 2010;101:1199-203. [PubMed]
- Troch M, Kiesewetter B, Raderer M. Recent developments in nongastric mucosa-associated lymphoid tissue lymphoma. Curr Hematol Malig Rep 2011;6:216-21. [PubMed]
- Cheah CY, Campbell BA, Seymour JF. Primary breast lymphoma. Cancer Treat Rev 2014;40:900-8. [PubMed]
- Ryan G, Martinelli G, Kuper-Hommel M, et al. Primary diffuse large B-cell lymphoma of the breast: prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Ann Oncol 2008;19:233-41. [PubMed]
- Martinelli G, Ryan G, Seymour JF, et al. Primary follicular and marginal-zone lymphoma of the breast: clinical features, prognostic factors and outcome: a study by the International Extranodal Lymphoma Study Group. Ann Oncol 2009;20:1993-9. [PubMed]
- Ryan GF, Roos DR, Seymour JF. Primary non-Hodgkin’s lymphoma of the breast: retrospective analysis of prognosis and patterns of failure in two Australian centers. Clin Lymphoma Myeloma 2006;6:337-41. [PubMed]
- Avilés A, Delgado S, Nambo MJ, et al. Primary breast lymphoma: results of a controlled clinical trial. Oncology 2005;69:256-60. [PubMed]
- Lin Y, Guo XM, Shen KW, et al. Primary breast lymphoma: long-term treatment outcome and prognosis. Leuk Lymphoma 2006;47:2102-9. [PubMed]
- Swerdlow SH, Campo E, Harris NL, et al. eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Fourth Edition. Lyon: International Agency for Research on Cancer, 2008.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11). Ann Hematol 2011;90:401-8. [PubMed]
- Swerdlow SH, Quintanilla-Martinez L, Willemze R, et al. Cutaneous B-cell lymphoproliferative disorders: report of the 2011 Society for Hematopathology/European Association for Haematopathology workshop. Am J Clin Pathol 2013;139:515-35. [PubMed]
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768-85. [PubMed]
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases. J Am Acad Dermatol 2011;65:749-55. [PubMed]
- Hallermann C, Niermann C, Fischer RJ, et al. Survival data for 299 patients with primary cutaneous lymphomas: a monocentre study. Acta Derm Venereol 2011;91:521-5. [PubMed]
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol 2013;69:357-65. [PubMed]
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood 2008;112:1600-9. [PubMed]
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi149-54. [PubMed]


