Upcoming innovations in lung cancer immunotherapy: focus on immune checkpoint inhibitors
Introduction
Lung cancer is one of the leading causes of cancer-related mortality worldwide. It has been categorized into non-small cell carcinoma (NSCLC) and small cell carcinoma (SCLC). SCLC has an aggressive clinical behavior which is characterized by a rapid growth and early metastasis. Although most SCLC patients respond very well to chemotherapies, they present a very high relapse rate and the long-term overall survival (OS) remains poor. NSCLC, on the other hand, represents about 85% of lung cancers and includes squamous cell carcinoma and adenocarcinoma histological subtypes. Squamous cell carcinoma is more closely associated with smoking than adenocarcinoma, which is often driven by activating mutations in oncogenes such as EGFR, KRAS, HER2, BRAF, etc.. Most patients are unfortunately diagnosed in a late advanced stage with a locally advanced or metastatic disease (stage IIIB or IV), when no treatment with curative intent is available. Until recently, the median survival of these patients was about 9-12 months with the use of conventional chemotherapy. Especially for patients with an oncogenic driven adenocarcinoma, the advent of molecular targeted therapies has increased the OS (to up to more than 2 years) and quality of life. However long-term survivors were rare and the benefits in OS were limited. Even for early diagnosed patients who underwent complete resection with curative intention, the 5-year survival rate was only around 40% (1). So there is a high unmet medical need for the treatment of lung cancer.
Recent successes with immune checkpoint blockade therapy, such as anti-CTLA4 and anti-PD1/PDL1 monoclonal antibodies (mAbs), demonstrated that manipulation of the immune system is a very potent way to fight cancer (2).
The immune system in cancer
A central problem in oncology is the reduced ability of the immune system to overcome an established tumor. Many studies reported that an effective cancer microenvironment induced immunosuppression that prevented cytotoxic T cells from killing the tumor cells. Immunosuppression in the microenvironment is mainly induced by regulatory T cell (Tregs), myeloid derived suppressor cells (MDSCs) and tumor activated macrophages (TAMs). These cells prevent the activation of anti-tumor T cells through different mechanisms including production of immunosuppressive cytokines, such as IL-10 and TGFb, and expression of co-inhibitory molecules, like CTLA4, PD1 and PDL1. Additionally, cancer cells can hide from the immune system by a diminished expression of major histocompatibility complex one (MHC-I) molecules, and therefore lower presentation of tumor-antigens to immune cells.
Immunotherapeutic approaches
Immunotherapies consist of a broad class of therapeutics that are designed to either provide immune effectors (passive immunotherapies; e.g., tumor targeted antibodies) or activate the patients’ immune cells (active immunotherapies; e.g., cytokines) in order to mediate the destruction of tumor cells. Interestingly, immune checkpoint targeted mAbs are an active immunotherapy. They stimulate the patient’s own immune system in order to destroy cancer cells. This is a paradigm shift in the treatment of cancer as opposed to conventional chemotherapies or tumor-targeted therapies that were designed to focus directly on the tumor cells.
Immunotherapeutics for lung cancer
Recent discoveries in the lung cancer field highlighted the mechanisms underlying tumor escape from the immune recognition. As for other cancer types, the presence of different effector T cells subsets seemed to be correlated with long-term OS (3,4). Beside the role of effector cells in antitumor biology in lung cancer, the presence of immunosuppressive cells such as MDSCs was correlated with a bad prognosis (5). Additionally, it was shown that Tregs from lung cancer patients could potentially inhibit autologous T cells activity (6). These data at least suggested that immune cells play a pivotal role in the pathogenesis of lung cancer.
Immunomodulatory agents
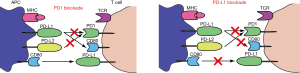
There are different classes of immunomodulatory agents: immune checkpoint modulators (anti-CTLA4, anti-PD1 and anti-PDL1), TLR agonists, cytokines, … (Table 1, Table S1). The hypothesis is that immune checkpoint inhibitors will activate the immune system by blocking the expression of co-inhibitory molecules such as CTLA4, PD1 and PDL1 (Figure 1), and as such re-activate the preexisting tumor response. Additionally, there is a strong preclinical rationale for a beneficial effect of agonistic antibodies against other activating co-stimulatory molecules such as OX40, LAG3 and GITR. Preclinical settings showed that antibody derived cellular cytotoxicity (ADCC) played a major role in the outcome of immunomodulatory antibody treatments such as anti-CTLA4 and anti-GITR (17). This means that CTLA4 expressing cells, mostly Tregs, will be killed by natural killer cells or macrophages, after binding to anti-CTLA4 (18). ADCC will depend on the Fc portion of the antibody, which differs according to the antibody IgG subtype. However this observation remains to be proven in patients.
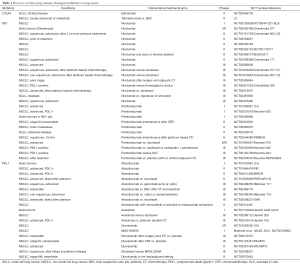
Full table

Full table
Monotherapy of immune checkpoint modulators (Table 1)
CTLA4 inhibitors
Ipilimumab and tremelimumab are both fully humanized anti-CTLA4 mAbs (Table 2), which block the binding of CTLA4 to its ligands (the co-stimulatory molecules CD80 and CD86). Two studies (NCT01165216, NCT00527735) in NSCLC patients showed that ipilimumab slightly improved the progression free survival (PFS) and the OS when given after chemotherapy in treatment-naive stage IV NSCLC patients (19,20). A small phase II trial with tremelimumab did not show any benefit as a maintenance therapy after first line chemotherapy (7).

Full table
PD1 and PDL1 inhibitors
Currently, two different anti-PD1 molecules (nivolumab, pembrolizumab) and four anti-PDL1 molecules (BMS-936559, atezolizumab, durvalumab and avelumab) are being tested in lung cancer patients (Table 2). Although anti-PDL1 and anti-PD1 may inhibit slightly different binding partners (Figure 1), it is too early to determine clinical differences between these molecules. Both nivolumab and pembrolizumab have been recently approved for melanoma by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) (21,22). The results of the phase I trial with nivolumab showed for the first time long term beneficial effects of anti-PD1 in lung cancer (8,9). Heavily pretreated patients had a 17% objective response rate, a median response duration of 74 weeks, and an ongoing response in more than half of the patients. Nivolumab was very well tolerated as only 14% experienced of the patients grade III or IV toxicity rates, and three therapy-related deaths were reported (pneumonitis). These cases of pneumonitis incited physicians to treat pneumonitis or other immune related side effects earlier with corticoids, which seemed to stop clinical aggravations. These results were confirmed in the recently published phase II trial with nivolumab (Checkmate 063): 14.5% of the patients had an objective response of which most is still ongoing and additionally 26% had stable disease (10). So in contrast to chemotherapy and most molecularly targeted therapies, and in analogy to the responses seen in metastatic melanoma patients, therapy responses are much more long-lasting which is paradigm changing for NSCLC. These revolutionary data were also seen in two recently published phase III trials: Checkmate 057 for non-squamous NSCLC and Checkmate 017 for squamous NSCLC. Both studies compared nivolumab to docetaxel and showed a response rate for nivolumab of about 20% versus about 10% for docetaxel with again long lasting responses. Additionally nivolumab was better tolerated than docetaxel (about 10% grade III-IV toxicity with nivolumab compared to 50% with docetaxel). An ongoing first-line phase III study is also conducted, comparing chemotherapy of choice with nivolumab in PDL1 positive patients (NCT02041533). The response to pembrolizumab in NSCLC corroborated the results from the nivolumab trials as it demonstrated an overall response rate (ORR) of 24% in patients who had failed two previous therapy regimen (13,23). Interestingly, these responses towards pembrolizumab seemed to correlate with a mutation burden and smoking history (24). Although further analysis is underway, mutational burden may be a good predictive biomarker for immunotherapy. Additionally, Keynote 010, a phase III trial comparing pembrolizumab vs. docetaxel, confirmed the superiority of checkpoint inhibition in treating NSCLC patients with PDL1 positive tumour cells (25). These positive results in a refractory patient group are very promising, prompting different phase III trials in second-line treatment comparing standard chemotherapy versus several anti-PD(L)1 antibodies (NCT02220894, NCT02142738). These paradigm changing results with both nivolumab and pembrolizumab have led to the US-FDA and the EMA approvals of nivolumab for patients with metastatic squamous cell lung cancer, refractory to platinum derivatives (21,22) and the US-FDA approval of pembrolizumab for PDL1 positive NSCLC (26).
The results with atezolizumab, an anti-PDL1 antibody (IgG1 but transformed not to induce ADCC of PDL1 expressing cells), in NSCLC affirm our enthusiasm: 23% of all NSCLC patients have shown a response in addition to 34% who had stable disease (NCT01375842) (14). These data were confirmed in the phase II POPLAR trial (NCT01903993) where it was shown that atezolizumab had an advantage over docetaxel in second line (especially in PDL1 high patients) (15). These data will be further analyzed in the OAK trial (phase III, NCT02008227). Two other anti-PDL1 antibodies (BMS 936559 and durvalumab) have demonstrated their efficacy in the phase I trials with NSCLC patients (NCT00729664, NCT01693562) (16,27). Now, almost 15 phase III trials are ongoing, challenging standard regimen in lung cancer patients: comparison between anti-PDL1 with standard chemotherapy (NCT02395172, NCT02008227), introduction of anti-PDL1 after chemoradiotherapy for stage III NSCLC patients (NCT02125461), introduction of anti-PDL1 in the adjuvant setting after complete resection in NSCLC patients (NCT02273375) among others.
Results from some clinical trials [e.g., Keynote 001 (23), Poplar (15)] may suggest that expression of PD-L1 on tumor-infiltrating immune cells or tumor cells may potentially be used as a biomarker to predict anti-tumor response to PD-1/PD-L1 inhibitors. Other trials especially with nivolumab (11,12) did not show a clear correlation. However at present, PDL1 staining is not standardized due to heterogeneities of staining, analysis, and timing, among the different industrial partners. Consequently, PDL1 positivity is not a good biomarker.
Combination therapy with immune checkpoint modulators (Table 3)
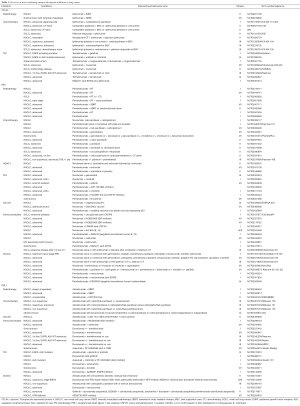
Full table
Although these results are very encouraging, monotherapy with checkpoint inhibitors still fail to induce a response in the majority of the metastatic patients. Preclinical research has shown that primary or secondary resistance to single immune checkpoint blockade could be overcome by targeting other immunosuppressive pathways or by combining immune checkpoint inhibitors. Very positive results were already shown in the melanoma field where combining ipilimumab with nivolumab induced response rates in up to 50% of the patients (34). Another strategy includes combinations with conventional treatments that induce antigen release due to cancer cell death (35-37). Therefore, many ongoing clinical trials are investigating combinations between immune checkpoint inhibitors, chemotherapy, radiotherapy, anti-angiogenic and targeted therapies (Table 3). Up to now, the results are eagerly awaited as only very few data from ongoing phase I trials have been released. The first results from Checkmate 012 (NCT01454102) and Keynote 021 (NCT02039674), both multi-arm studies which combine anti-PD1 (nivolumab and pembrolizumab) with either chemotherapy, bevacizumab, erlotinib or ipilimumab are very promising. Combining pembrolizumab with ipilimumab could induce response rates of about 60% of the patients (31), and this robust anti-tumour response was confirmed with the combination of pembrolizumab with ipilimumab that showed responses in about 40% of the patients. Compared to the first trials in melanoma the toxicity of the combination could be largely reduced by using intervals of e.g., 12 weeks for ipilimumab. These are very early data that should be interpreted with caution but it clearly demonstrates that combining immune checkpoint inhibitors may be a very powerful therapy in lung cancer. Trials with such combinations in NSCLC (anti-CTLA4 antibodies with anti-PDL1 antibodies) are ongoing [ARTIC (NCT02352948) and Neptune (NCT02542293)]. Even in advanced SCLC patients a combination of ipilimumab and nivolumab could induce long lasting responses in about 25% of the patients (NCT01928394) (29). Checkmate 451 (phase III, NCT02538666) will further analyze the combination of nivolumab with ipilimumab in early stage SCLC after platinum therapy.
Another possible synergistic combination consists of chemotherapy plus checkpoint inhibition. Also here, some early results are very encouraging: the combination of platinum doublets with pembrolizumab induced response rates of about 60% (Keynote 012) (32) and similar results were seen in the phase Ib trial that used a combination of atezolizumab with several chemotherapeuticals (NCT01633970) (33). Many phase III trials combining chemotherapy with immune checkpoint inhibition are underway (NCT02578680/Keynote-189, NCT02477826/Checkmate 227, NCT02367781/IMpower 130, NCT02366143, …).
Cytokine therapy
Interleukin-2 (IL-2) induces T cells activation and proliferation. It has a long history in melanoma and renal cancer as a successful therapy with durable responses. However, only a minority of patients respond and it is suitable for very few patients due to a high toxicity profile (38). This approach has also been tested in several trials designed for lung cancer patients. However, most of them have failed to show any superiority compared to conventional treatments. Recently a phase I trial has been conducted analyzing the combination of an alternative IL-2 treatment [NHS-IL2 selectikine: human NHS76 (antibody specific for necrotic DNA) fused to genetically modified human IL-2] with local irradiation of a single pulmonary nodule (5×4 Gy) in metastatic NSCLC patients that had stable disease after one line of chemotherapy (39). Beside a possible therapy related thyroiditis in two patients, there were no major side effects. Two out of 13 patients had a long-term response (over 4 years). Thus, the efficacy of this treatment needs to be confirmed in further trials.
Anti-IDO vaccination
A Danish group from Copenhagen has reported a phase I trial with a very original approach by targeting an immunosuppressive enzyme indoleamine 2,3 dioxygenase (IDO). Because they had previously shown that anti-IDO T cells may exist in human beings, they wanted to boost this anti-inhibitory Tcells with a vaccine consisting of an IDO peptide. The ultimate goal is to setup anti-IDO T cells that should have a boosting effect on the immune system. In the 15 treated patients with NSCLC, no toxicity was seen and there was a median OS of 25 months which was superior to the 7 months for the non-vaccinated group (excluded because not HLA-A2 positive) (40). These interesting results need confirmation in a double blind trial.
Vaccine treatments (Table S2)

Full table
The aim of a vaccine treatment in tumor biology is to mount an effective immune response by the host in order to eliminate the cancer cells by specific recognition by cytotoxic CD8 T cells. However, in contrast to healthy subjects, cancer patients are already in an immunosuppressed status. So it might be even more difficult to find an effective vaccination strategy in a cancer patient. Different types of vaccines exist: rather aspecific whole tumor or tumor lysate vaccination versus strategies where one epitope of a tumor antigen is the target. The latter strategy includes RNA and DNA vaccines, specific gangliosides or peptide vaccines and autologous dendritic cell vaccines. The actual timing of a vaccine in the anti-cancer treatment is mostly in the adjuvant setting: after successful surgery accompanied with or without chemotherapy. In general and despite many efforts in lung cancer research, therapeutical vaccinations did not lead to a breakthrough [reviewed by (41)]. Nevertheless, many studies found a niche or a genetic signature of patients who might benefit from a vaccine (Table S2). The reason for these failures might reside on the fact that these vaccines did not address the issue of tumor antigen tolerance in cancer patient. Indeed, cancer cells are “self” cells and have therefore multiple pathways to hamper immune cells from attacking them. Therefore, it might be very interesting to combine a vaccine with an immune checkpoint modulator, as CD8 T cell response might be boosted directly via the vaccine and indirectly by relieving immunosuppression. This strategy is supported by a lot of preclinical rationale and should soon be tested in lung cancer patients. Some trials have already been set up (NCT NCT02495636, NCT02466568, …Table S2).
Adoptive T cell therapy approaches (Table S3)

Full table
In order to evade the formation of an effective CD8 T cell response upon vaccination, one step further is to inject directly tumor specific T cells. Most approaches hereby consist in isolating T cells from the initial tumor, let them exponentially grow and reinfuse them in much larger quantities into cancer patients. This strategy was very effective in a well selected population with metastatic melanoma (42) and is ongoing in lung cancer (NCT00569296, NCT02133196). Another T cell based strategy is the use of CAR T cells. These T cells are transfected with a transformed hybrid molecule between a single chain Fv directed against a tumor antigen and a T-cell receptor intracellular signaling. Such CAR T-cells can directly recognize a tumor antigen without the need of MHC molecules. This strategy turned out to be very effective in CD19 malignancies with CD19 CAR T cells (43). A similar strategy using CARs directed against VEGFR2 is underway in lung cancer patients (NCT01218867).
Conclusions and future perspectives
One of the major challenges in the field of lung cancer immunotherapy is to overcome primary and secondary resistance towards anti-PD(L)1 antibody therapy. Preclinical research showed that a combination of checkpoint inhibition with radiotherapy, chemotherapy, anti-angiogenesis, and targeted therapy, could increase the cure rate (44). However early results from a combination of TKI and checkpoint inhibition in renal cancer (45) did show major liver toxicity, slowing down the development of these combinatorial regimens. Recently, two trials combing a TKI with durvalumab were suspended due to interstitial lung disease (46). Therefore innovating trials with different timing of the treatments (sequential, alternating days) or different administration routes (e.g., intratumoral) are highly awaited. The first results of the combination of nivolumab and ipilimumab showed increased OS rates both in patients with NSCLC and SCLC with manageable toxicity (29,31). About 20 trials have started to address this question (Table 3). Preclinical studies combining radiotherapy with checkpoint inhibition showed increased occurrence of abscopal effect [tumor response outside radiotherapy field due to immunological induced anti-cancer response (47)]. Therefore, up to 12 trials combining immune checkpoint blockade with radiotherapy are under way (Table 3), including trials conducted at our institute (personal communication Dr. Deutsch). Up to now, only very disperse results on abscopal effect in patients were reported (48). Another promising strategy is to combine chemotherapy with immunotherapy. However, as chemotherapy may be deleterious for lymphocytes, this may diminish the formation of an immunological memory, which is important for long term immunological memory (20 trials ongoing Table 3). Last but not least, several preclinical studies suggest that it could be very interesting to repeat some of the vaccination studies with our current knowledge of immune checkpoint inhibitors, even if our clinical experience with the combination of gp100 with ipilimumab did not confirm this hypothesis (49). Several studies are ongoing to evaluate if PD1 antibodies have a better profile (NCT02439450, NCT02466568, NCT02432963). In addition to the results of the ongoing clinical trials that evaluate the feasibility and efficacy of these combinations, the results of the ancillary studies are of interest to find novel biomarkers in responding patients, in order to tailor immunotherapy to each patient and diminish side effects. Therefore are awaiting highly interesting times in the field of immuno-oncology.
Acknowledgements
Sandrine Aspeslagh is an ESMO fellow (Georges Mathé grant 2014).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rosell R, Skrzypski M, Jassem E, et al. BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS One 2007;2:e1129. [PubMed]
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013;342:1432-3. [PubMed]
- Zikos TA, Donnenberg AD, Landreneau RJ, et al. Lung T-cell subset composition at the time of surgical resection is a prognostic indicator in non-small cell lung cancer. Cancer Immunol Immunother 2011;60:819-27. [PubMed]
- Djenidi F, Adam J, Goubar A, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol 2015;194:3475-86. [PubMed]
- Huang A, Zhang B, Wang B, et al. Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother 2013;62:1439-51. [PubMed]
- Woo EY, Yeh H, Chu CS, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 2002;168:4272-6. [PubMed]
- Zatloukal P, Heo DS, Park K, et al. Randomized phase II clinical trial comparing tremelimumab with best supportive care following first-line platinum-based therapy in patients with advanced non-small cell lung cancer. J Clin Oncol 2009;27:abstr 8071.
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [PubMed]
- Garon EB, Leighl NB, Rizvi NA, et al. Safety and Clinical Activity of Pembrolizumab (MK-3475) in Previously Treated Patients With Non-Small Cell Lung Cancer. J Clin Oncol 2014;32:abstr 8002.
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [PubMed]
- Spira A, Park K, Mazières J, et al. Efficacy, safety and predictive biomarker results from a randomized phase II study comparing MPDL3280A vs docetaxel in 2L/3L NSCLC (POPLAR). J Clin Oncol 2015;33:abstr 8010.
- Khleif SN, Lutzky J, Segal NH, et al. MEDI4736, an anti-PD-L1 antibody with modified Fc domain: preclinical evaluation and early clinical results from a phase I study in patients with advanced solid tumors; Proceedings from the European Cancer Congress 2013; 2013 Sep 27-Oct 1; Amsterdam, The Netherlands. Abstract No. 802.
- Bulliard Y, Jolicoeur R, Windman M, et al. Activating Fc receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med 2013;210:1685-93. [PubMed]
- Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013;210:1695-710. [PubMed]
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [PubMed]
- Horinouchi H, Yamamoto N, Fujiwara Y, et al. Phase I study of ipilimumab in phased combination with paclitaxel and carboplatin in Japanese patients with non-small-cell lung cancer. Invest New Drugs 2015;33:881-9. [PubMed]
- FDA expands approved use of Opdivo to treat lung cancer. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm436534.htm
- Opdivo. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003985/human_med_001876.jsp&mid=WC0b01ac058001d124
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2015. [PubMed]
- FDA approves Keytruda for advanced non-small cell lung cancer. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm465444.htm
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [PubMed]
- Antonia S, Bendell J, Matthew T, et al. Phase I/II Study (CheckMate 032) of Nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC). J Clin Oncol 2015;33:abstr 7503.
- Rizvi N, gettinger S, Goldman JW. Safety and efficacy of first-line nivolumab and ipilimumab in non-small cell lung cancer. World Conf Lung Cancer. 2015:abstr orla 02.05.
- Patnaik A, Socinski M, Gubens M, et al. Phase 1 study of pembrolizumab (pembro; MK-3475) plus ipilimumab (IPI) as second-line therapy for advanced non-small cell lung cancer (NSCLC): KEYNOTE-021 cohort D. J Clin Oncol 2015;33:abstr 8011.
- Papdimitrakopoulou V, Patnaik A, Borghaei H, et al. Pembrolizumab (pembro; MK-3475) plus platinum doublet chemotherapy (PDC) as front-line therapy for advanced non-small cell lung cancer (NSCLC): KEYNOTE-021 Cohorts A and C. J Clin Oncol 2015;33:abstr 8031.
- Liu S, Powderly JD, Camidge DR, et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with platinum-based doublet chemotherapy in patients with advanced non-small cell lung cancer. J Clin Oncol 2015;33:abstr 8030.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [PubMed]
- Marabelle A, Kohrt H, Sagiv-Barfi I, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest 2013;123:2447-63. [PubMed]
- Brody J, Kohrt H, Marabelle A, et al. Active and passive immunotherapy for lymphoma: proving principles and improving results. J Clin Oncol 2011;29:1864-75. [PubMed]
- Le Mercier I, Poujol D, Sanlaville A, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res 2013;73:4629-40. [PubMed]
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014;192:5451-8. [PubMed]
- van den Heuvel MJ, Garg N, Van Kaer L, et al. NKT cell costimulation: experimental progress and therapeutic promise. Trends Mol Med 2011;17:65-77. [PubMed]
- Iversen TZ, Engell-Noerregaard L, Ellebaek E, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res 2014;20:221-32. [PubMed]
- Declerck S, Vansteenkiste J. Immunotherapy for lung cancer: ongoing clinical trials. Future Oncol 2014;10:91-105. [PubMed]
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62-8. [PubMed]
- Maus MV, Grupp SA, Porter DL, et al. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014;123:2625-35. [PubMed]
- Antonia SJ, Larkin J, Ascierto PA. Immuno-oncology combinations: a review of clinical experience and future prospects. Clin Cancer Res 2014;20:6258-68. [PubMed]
- Amin A, Plimack E, Infante J, et al. Nivolumab (anti-PD1) in combination with sunitib or pazopanib in patients with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2014;32:abstr 5010.
- AstraZeneca PLC (AZN) Halts Two Lung Cancer Drug Combination Trials After Lung Disease Reports. Available online: http://www.biospace.com/News/astrazeneca-plc-halts-two-lung-cancer-drug/394464
- Frey B, Rubner Y, Wunderlich R, et al. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation - implications for cancer therapies. Curr Med Chem 2012;19:1751-64. [PubMed]
- Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013;1:365-72. [PubMed]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]




