Autologous tracheal replacement for cancer
Indications
Tracheal cancer is a relatively rare occurrence. Tracheal tumors as a whole represent 0.2% of respiratory malignancies and most of these are squamous cell carcinomas (SCC). Only 10% of these cases are adenoid cystic carcinoma (ACC). The primary management of tracheal ACC is surgical resection. In the unresectable ACC patients, radiotherapy alone was recommended, with an overall survival of 30% (1).
It is generally agreed that the maximum length of tracheal resection that can be repaired by end to end anastomosis is 6 cm (2). Primary tracheal neoplasms (including ACC and SCC) and other tracheal diseases can be usually managed by tracheal resection with primary anastomoses. However, there are diseases that require resection of segments of trachea longer than 6 cm that require reconstruction with a tracheal substitute (3). All the prostheses used to replace the trachea could lead to erosion of the adjacent structures, infection and obstructive issues (4-6). En bloc transplantation of the trachea is a technical solution but do require immunosuppressive therapy (7). Bioengineering and transplantation of the trachea seems to be a promising technique but is not actually accurate for such extended diseases (8,9).
Tracheal replacement for disease invading 6 to 12 centimeters of the trachea can be performed with the use of reliable autologous free fasciocutaneous flaps in combination with autologous cartilage struts (10-12). Previously, circumferential resection with release procedures (suprathyroid or suprahyoid laryngeal release) and direct anastomosis must be considered before this procedure. The most reliable flap for this reconstruction is the forearm free flap (FFF) that could replace the entire membranous wall of the trachea for extended tracheo-esophagal fistulae (TOF). Reinforced with cartilage ribs this autologous tracheal substitution (ATS) using the FFF can replace the entire trachea from the cricoid cartilage to the carena. Indications are primary tracheal neoplasm (including ACC and SCC), secondary tracheal neoplasm (including thyroid carcinoma, thymic carcinoma, …) and extended TOF (post-intubation tracheal destruction, tracheal necrosis after lymphoma, …). Benign and malignant neoplasms have to be discussed by a multidisciplinary team before surgery.
Contraindications
Determination of resectability
Tracheal resection was considered when complete resection of gross airway disease appeared feasible. An assessment of locoregional and distant metastatic disease has to be performed before resection (2,3). Preoperative radiation therapy (RT) is associated with a higher incidence of complications but is not a contraindication for ATS. Bronchoscopy has to be performed before resection to assess the presence and extent of luminal invasion. Patients with SCC and N2 disease should be contraindicated for ATS. N2 disease is assessed using preferentially EBUS, EUS or mediastinoscopy. Bilateral recurrent nerve involvement is most of the time an indication for laryngectomy. Involvement up to the cricoid or thyroid cartilage and extended esophagal involvement are considered as contraindications to this extensive and difficult surgery. But a partial laryngotracheal resection could be performed in few cases. On bronchoscopy, the length of involved airway precluded. The length for tracheal reconstruction is limited to 12 centimeters. Tracheal tumor extension to the carena is currently a contraindication for ATS. Indeed, the main limitation of the neo-trachea is the absence of mucociliary clearance because of its inner aspect is covered by a squamous epithelium. The quality of the mucociliary clearance is correlated to the resection length. We currently do not recommend this technic to treat lesions that extend to the main bronchi or for patients with pulmonary and diaphragmatic dysfunction of sufficient magnitude to interfere with effective coughing. The limits of this technique are also chronic respiratory insufficiency and cartilage calcifications (risk of cartilage fracture).
Preoperative planning
Patient selection
Because of the high risks of this surgery a careful preoperative assessment of each patient is required. Ethics approval should be granted by the Ethics Board, and individual patient consent should be obtained. For neoplasms, a multidisciplinary consultation (including thoracic oncologist and thoracic surgeon) before surgery is needed to confirm the indication for ATS. Patients with an extended primary tracheal neoplasm, a secondary tracheal neoplasm or an extended tracheal destruction should are the main indication for this ATS. Indication for ATS in case of ACC with lung metastasis should be carefully weighted by the actual results of RT.
Patients should be carefully screened from a general medical point of view. The age, sex, histology, preoperative medical history, pulmonary function test, performance, laboratory tests, tumor location, vocal cords function, and cardiac function should be carefully assessed. The diagnostic staging modalities included bronchoscopy, computed tomography (CT) scanning, and positron emission tomography-CT (PET-CT). Echocardiography and stress thallium are used when indicated. Angiography of supra aortic arteries should be performed to warrant the patency of the donor vessel for microvascular anastomosis of the free flap. Furthermore involvement and patency of the supra-aortic arteries has to be assessed before surgery. Allen’s test must be performed on both sides (the color of the hand should return to normal in 7 seconds) to confirm the possibility for FFF harvesting. Predicted postoperative forced expiratory volume in 1 second should be more than 70%. Indeed, respiratory failure is a contraindication for ATS due to the increasing pressure on the neotracheal wall during inspiratory depression. Indeed, respiratory insufficiency can lead to late cartilage fracture in the free flap.
Surgery
Subheading for surgery
The patient is positioned supine, anesthetized and intubated with a single lumen endotracheal tube. An inflatable bag beneath the patient’s shoulders is very useful. For this long operation two teams of surgeons are working together. The entire chest and entire neck is prepped. For the FFF harvesting, the arm should be placed on an arm table usually placed at 90 degrees. ATS is performed by an open approach through a transverse cervicotomy with a median vertical sternotomy. The groin could be used for cannulation and one of the thigh should be prepared for skin graft on the harvested arm. The cartilage harvesting is usually performed on the opposite site from the free flap harvesting.
The first part of the surgery is the tracheal resection including firstly determination of local respectability. After this part performed by the thoracic team, the reconstruction team can start the flap harvesting. During this the cartilages could be harvested on the contralateral side.
Tumor resection
Before completing the construction of the neo-tracheal conduit, the damaged trachea is approached by cervicotomy and median sternotomy. The diseased trachea is resected on healthy margins and the autologous conduit sutured to the native tracheal stumps (i.e., to the tracheobronchial bifurcation below and to the larynx above). If necessary, in addition to removing the trachea, partial or total resection of the esophagus and removal of one of the recurrential nerves, the innominate vein is not transected. During this stage of the procedure, respiratory gas exchange is provided by ventilation with an intubation tube inserted into the bronchi through the surgical field. Alternatively, extracorporeal circulation (ECC) between the right atrium and the ascending aorta can be instituted. At the end of the reconstruction, an extended lymph node resection is performed before ATS reconstruction.
ATS construction
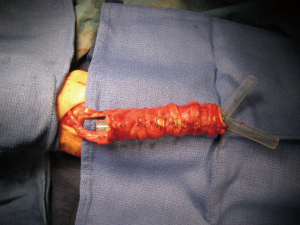
The neo-tracheal conduit is constructed from a large, rectangular fasciocutaneous flap harvested from the patient’s forearm. The skin of this flap is rotated around a silicone tube, the diameter of which is approximately slightly larger than that of the normal trachea. The tubular conduit, supplied by the radial artery and vein, is made of skin along its inner aspect and of fascia in its outer aspect. Before transforming the fasciocutaneous rectangle of the FFF into a tube by suturing the main lengths together, several costal cartilage segments obtained from the patient’s rib cage have to be inserted between skin and fascia in the subcutaneous tissue to ensure the transverse rigidity of the tube. Through a transverse latero-sternal approach, the cartilage junction of the fifth sixth and seventh ribs is exposed. Typically, 6 or 7 cartilaginous segments (5 mm in width, 2 mm in thickness and about 9 cm long) are elevated from the most caudal ribs and then slid in place taking care at not injuring the skin vascular supply from the perforators. Transillumination should be used to locate the perforating vessels. A tunnel is created for each cartilage just under the skin with Metzenbaum scissors. Halstead forceps are used to insert the cartilages ribs in all the different tunnels. The flap is then rolled around a silicone stent (Tracheobronxane® Dumon silicone stent Novatech). We recommend to use a simple straight tube or a Y tube when the distal tracheal suture is close to the carina. The caliber should be preoperatively sized up based on the CT scan. The structural rigidity of the neo-tracheal “cylinder” is then obtained by suturing the skin around the stent and the ends of each costal cartilage between each other so that this framework is included in the wall of the conduit without coming into contact with the lumen of the trachea. During the phase of construction of this totally autologous neo-trachea the flap remains vascularized by its radial pedicle dissected up to the level of the elbow (Figure 1). This pedicle will be divided only immediately after its final implantation in the chest cavity to minimize the flap ischemic time. The last step is to suture the fascia of the FFF around the cartilages. This step is major to avoid mediastinal structures erosion. The fascia should be harvested to be enough large to ensure this cartilage coverage (1 centimeter on each side of the flap).
Tracheal replacement (Figure 2)
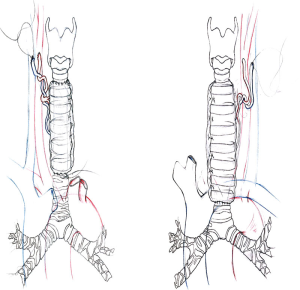
Revascularisation of the flap is provided by microanastomoses performed with a magnification microscope between the radial vessels of the flap and small neck vessels. The most frequently used arteries are the internal thoracic artery or branches of the subclavian artery or external carotid artery. The duration of microvascular anastomosis must be the shortest. Technically, the distal tracheal anastomosis has to be done firstly before the microvascular anastomosis. For this tracheal anastomosis, a silicone stent (preoperatively sized) is placed before performing the anastomosis and cut just to cover the level of the anastomosis. Both tracheal anastomoses are performed with a posterior continuous suture and an anterior interrupted suture. Careful calibration of the conduit proved necessary to counteract narrowing of the neo-tracheal lumen due to flap edema and high inspiratory negative pressures generated by bronchial congestion and laryngeal edema.
Intercricothyroid transitory tracheostomy has to be performed at the end of the procedure after closure of all incisions. A simple skin graft is harvested on the thigh with a dermatome to cover the forearm. A forearm splint cast is then applied for 21 days.
Postoperative management
Immediately postoperatively, patients are transported intubated to the intensive care unit (ICU) for constant monitoring. Follow-up visits may include the following: complete physical examination chest X-ray, blood gas, daily bronchoscopy through the tracheostomy,
Prior to extubation, patients undergo bronchoscopy to ensure adequate clearance of secretions. Following extubation, the chest tubes are removed in the absence of an air leak, commonly within 48 hours postoperatively. The cervical drainage is removed after negative bacteriological culture of the daily sample.
Temporary tracheotomy is recommended for all patients with a complete tracheal replacement. In addition to daily bronchoscopies, bacteriological sampling and postural drainage we also believe that the postoperative management should be performed by an experienced surgeon in tracheal surgery.
As an average, the airway stent has to be removed bronchoscopically after surgery usually one week after surgery. In all instances, the flap should be endoscopically checked once daily for satisfactory healing of the anastomoses and viability. Frequent bronchoscopies are needed to clear retained secretions. Transitory tracheotomy has to be associated due to absence of muco-ciliary clearance and a medical humidifier must be used. Patients should be followed and controlled with bronchoscopy at 1 month, 6 months, 1 year and every year. An adjuvant treatment has to be associated in case of R1 resection for ACC on the proximal and distal anastomosis.
Viability and collapsibility of the flap
Viability is usually easily assessed by bronchoscopy: color of the flap is the best indicator of viability. The anastomotic integrity is directly checked. All flaps should remain viable; the cartilaginous framework prevents inspiratory collapse. There is a late contrast enhancement of the cartilages and of the skin. Pedicles are controlled. CT reconstructions are performed to check the cartilages in the skin flap. In one patient, a late fracture of the cartilages appears with the need for tracheostomy.
The mean length of hospital stay is around one month. All patients operated on for ATS have to be early extubated but they all present at least one pulmonary infection leading in ARDS (50% of cases). Thereby daily bronchoscopic aspiration should be performed. Patients usually do not need any mechanical ventilation and breathe through the tracheostomy tube during the first days. But in case of ARDS, a mechanical ventilation is necessary. The risk of flap necrosis should be checked by daily bronchoscopy. In case of flap necrosis patients should be reoperated. Erosion of the posterior part of the innominate artery by the neotrachea has to be prevented by interposition of tissue.
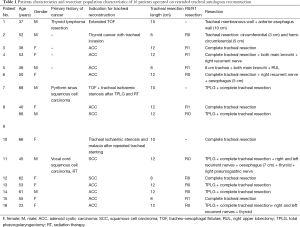
Results (Tables 1 and 2)
Full table

Full table
Between August 2004 and April 2015, we treated 16 consecutive patients who required ATS after resection of the trachea. They included 7 men and 9 women, with a mean age of 50.6 years (range from 37 to 68 years). The lengths of the tracheal involvement lead to mean length of tracheal resection of 10.2 cm (range from 8 to 12 centimeters). Indications were 12 primary tracheal neoplasms (including 9 ACC and 3 SCC), 3 secondary tracheal neoplasms (including 1 thyroid carcinoma and 2 tracheal lymphomas) and one post-intubation extended tracheal destruction after long history of stenting. Full-length resection from the first cartilaginous ring to the carina was required in 9 patients upon 16 with an extended circular tracheal resection (56.2%). When locally invaded, recurrent laryngeal nerve was resected but we did not observe any recurrent laryngeal nerve injury in our experience. For 2 patients, tracheal resection included the carina and both main bronchi; one of these patients also needed a right upper lobectomy (RUL). Total laryngectomy with ATS was performed in 6 cases with end stomas. Cardiopulmonary bypass (CPB) was used in 6 cases upon 12 in order to avoid intermittent ventilation of the left main bronchus in patient with extension to the carena. CPB is started before dividing the distal trachea and stopped just after performing distal tracheal anastomosis.
After ATS, only two patients who suffered from ARDS after replacement of the trachea and the main bronchi died after a long ICU stay. One of them was treated with a veno-venous ECMO. In both instances, the flaps remained viable and functional until the patients died from respiratory infection and excessive bronchial congestion. We did not observe any flap failure, even in one patient who was treated in emergency for a brachio-cephalic artery rupture
Ten patients are currently alive, without respiratory dysfunction and living normal lives. Two patients died of cancer recurrence at 6 months and of lung metastases at 16 months after surgery.
One patient with a chronic severe respiratory insufficiency required a distal and short stent and two others have a permanent tracheostomy. In one of them with a severe respiratory insufficiency the lumen collapse is related to a break of the calcified rings of costal cartilage in the other one at the level of the postoperative tracheostomy which was performed through the upper airway anastomosis.
The two patients who complained of dysphagia before the procedure due to extensive esophago-tracheal fistulas were able to resume oral feeding after surgery. Repeat endoscopy and dynamic CT scan demonstrated satisfactory patency of the neo-trachea without inspiratory collapse both during the immediate postoperative period, when the respiratory cycle increases the endoluminal negative pressure, as well as at several years after the procedure. The Kaplan-Meier survival analysis for 15 patients with cancer indicates a 64.8% of survival at 5 years.
Discussion
Firstly, we have actually alternative approaches such as modern chemoradiotherapy to treat ACC of the trachea. According to recent literature, chemoradiation is actually limited for patients who are unresectable (13).
The actual 5-year survival for patients treated with surgical resection is more than 50% (1) and the overall survival for unresectable patients is around 30%. Combination of treatment should be always considered to improve the long term survival (14).
Definition of unresectable patients could also be changed including ATS as an option to treat extended tumor of the trachea.
The primary management of such extended tumor is surgical resection and the level of morbidity and mortality that was high in the beginning of our experience is actually better. As reported by Wurtz et al., the benefit of ATS has actually improved with shorter post-operative course (15). But, as reported by Honings et al., the results of ACC with positive margins or locally advanced disease are good with long term survival up to 10 years (16).
The last important point is the anatomical restriction to standard anterior mediastinal tracheostomy. Indeed, the resection of less than 50% of the proximal the resection of the distal trachea could not be anastomosed to the anterior mediastinum due to the posterior mediatinal position of the carena. Also, when the larynx is sacrificed with more than 50% of trachea, ATS should be considered as an option (17).
Conclusions
ATS is a reliable tracheal substitute, totally autologous, well vascularized and resistant to infection. Transverselly rigid is enough to resist respiratory pressure variations. On the other hand because of the absence of a mucociliary clearance of the skin epithelium, transitory tracheostomy and postural drainage are mandatory. We have shown that extensive tracheal diseases previously not amenable to tracheal replacement can now be successfully managed with the use of totally autologous conduits made of fasciocutaneous free flaps reinforced by costal cartilages. This ATS has nearly all the qualities of the ideal tracheal substitute. The absence of a ciliated epithelial lining to provide mucociliary clearance remains a limiting aspect of this method. Whereas excellent results can be achieved with autologous neo-tracheal, fascio-cutaneous-cartilage supported autologous tube grafts, we currently do not recommend this method to treat lesions that extend to the main bronchi or for patients with pulmonary and diaphragmatic dysfunction of sufficient magnitude to interfere with effective coaching. Research to develop a method for lining the neo-trachea with ciliated respiratory epithelium is needed.
Acknowledgements
The authors would like to thank the “Gueules Cassées” Fundation and the “Union des Blessés de la Face et de la Tete” for the awards and financial support during the five last years.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg 2004;78:1889-96; discussion 1896-7.
- Pearson FG, Thompson DW, Weissberg D, et al. Adenoid cystic carcinoma of the trachea. Experience with 16 patients managed by tracheal resection. Ann Thorac Surg 1974;18:16-29. [PubMed]
- Grillo HC. Tracheal replacement: a critical review. Ann Thorac Surg 2002;73:1995-2004. [PubMed]
- Lenot B, Macchiarini P, Dulmet E, et al. Tracheal allograft replacement. An unsuccessful method. Eur J Cardiothorac Surg 1993;7:648-52. [PubMed]
- Wurtz A, Porte H, Conti M, et al. Surgical technique and results of tracheal and carinal replacement with aortic allografts for salivary gland-type carcinoma. J Thorac Cardiovasc Surg 2010;140:387-393.e2.
- Davidson MB, Mustafa K, Girdwood RW. Tracheal replacement with an aortic homograft. Ann Thorac Surg 2009;88:1006-8. [PubMed]
- Macedo A, Fadel E, Mazmanian GM, et al. Heterotopic en bloc tracheobronchial transplantation with direct revascularization in pigs. J Thorac Cardiovasc Surg 2004;127:1593-601. [PubMed]
- Delaere P, Vranckx J, Verleden G, et al. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med 2010;362:138-45. [PubMed]
- Elliott MJ, De Coppi P, Speggiorin S, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 2012;380:994-1000. [PubMed]
- Beldholm BR, Wilson MK, Gallagher RM, et al. Reconstruction of the trachea with a tubed radial forearm free flap. J Thorac Cardiovasc Surg 2003;126:545-50. [PubMed]
- Fabre D, Singhal S, De Montpreville V, et al. Composite cervical skin and cartilage flap provides a novel large airway substitute after long-segment tracheal resection. J Thorac Cardiovasc Surg 2009;138:32-9. [PubMed]
- Fabre D, Kolb F, Fadel E, et al. Successful tracheal replacement in humans using autologous tissues: an 8-year experience. Ann Thorac Surg 2013;96:1146-55. [PubMed]
- Allen AM, Rabin MS, Reilly JJ, et al. Unresectable adenoid cystic carcinoma of the trachea treated with chemoradiation. J Clin Oncol 2007;25:5521-3. [PubMed]
- Misiukiewicz KJ, Camille N, Tishler R, et al. Organ preservation for adenoid cystic carcinoma of the larynx. Oncologist 2013;18:579-83. [PubMed]
- Wurtz A. Circumferential tracheal replacement: do the benefits warrant the risks? Ann Thorac Surg 2014;97:1480. [PubMed]
- Honings J, Gaissert HA, Weinberg AC, et al. Prognostic value of pathologic characteristics and resection margins in tracheal adenoid cystic carcinoma. Eur J Cardiothorac Surg 2010;37:1438-44. [PubMed]
- Berthet JP, Garrel R, Gimferrer JM, et al. Anterior mediastinal tracheostomy as salvage operation. Ann Thorac Surg 2014;98:1026-33. [PubMed]