Subclavian artery resection and reconstruction for thoracic inlet neoplasms
Introduction
Extended resection procedures for locally advanced malignancies have been developed over the past few decades and shown to provide long-term survival (1-3) in situations that respond poorly to exclusive chemo-radiation therapy. The thoracic inlet (TI) is a small region traversed by major vessels and nerves, the esophagus, and the trachea, which are therefore often invaded by TI malignancies. Hence, surgical resection of TI tumors is challenging.
We previously described an anterior approach to the TI providing safe exposure of the subclavian vessels, brachial plexus, and apical chest wall and allowing complete resection of TI malignancies in selected patients, followed by long-term survival (4). Involvement of the subclavian artery (SA) is independently associated with lower long-term survival rates after surgical resection of TI malignancies (5). However, resection and reconstruction of the involved SA does not seem to increase the postoperative morbidity or mortality rates after surgical resection of TI malignancies (6). Therefore, applying modern principles of vascular surgery to SA resection and reconstruction during resection of TI malignancies might provide long-term survival and might constitute an alternative to conventional chemoradiotherapy in carefully selected patients (5-8). We have already shown that surgical management of tumoral involvement of the SA could achieve long-term survival (i.e., 22% at 5 years) in such selected patients (9).
The objectives of this study are to update our experience with SA resection and reconstruction during surgical resection of TI malignancies through the anterior transclavicular approach and to evaluate long-term outcomes after this procedure.
Patients and methods
Between August 1985 and November 2014, 85 patients underwent en bloc SA resection for TI malignancies in our department. There were 60 men and 25 women (female/male ratio, 2.4) with a mean age of 52 years (range, 17–80 years). Their medical records were reviewed retrospectively for collection of the following data: clinical assessment, tumor histology, characteristics of surgery, clinical course, and survival. Our institutional review board approved this retrospective study and waived the need for informed consent.
Diagnosis of subclavian artery (SA) invasion by thoracic inlet (TI) malignancies
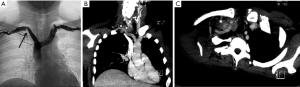
All patients presented with Pancoast syndrome caused by malignancies invading the TI and manifesting as severe shoulder pain radiating to the axilla, scapula, and hand, combined with Horner syndrome. There were no specific symptoms of SA invasion such as upper limb ischemia, claudication, subclavian steal syndrome, or digital ischemia. SA involvement was suspected by duplex ultrasonography and confirmed by preoperative thoracic computed tomography (CT) and angiography (Figure 1). Venous angiography was used to look for subclavian vein involvement and magnetic resonance imaging (MRI) for spinal involvement. When needed, MRI was extended to the brachial plexus to rule out invasion higher than the C8 root. The preoperative pathological diagnosis was obtained by either CT-guided percutaneous biopsy or surgical biopsy. There were 74 carcinomas and 11 sarcomas (Table 1).
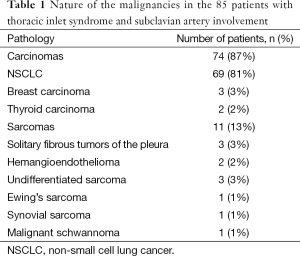
Full table
The comprehensive workup included functional testing (spirometry, ventilation/perfusion scan, and echocardiography), bronchoscopy, and imaging for staging [18F-fluorodeoxyglucose positron emission tomography (FDG-PET) and cerebral CT or MRI].
Contraindications to surgical resection of TI malignancies were substantial involvement of the esophagus, trachea, and/or brachial plexus higher than the C8 root; distant metastasis; and clinical N2 or N3 carcinoma as determined by the preoperative workup (5).
Surgical technique
All patients underwent one-stage en bloc resection of the TI malignancy through an anterior transcervical approach as previously described (4,9). Briefly, the surgical approach was performed through an L-shaped cervicotomy. The inner part of the clavicle was removed if the tumor was deemed resectable. Involvement of the subclavian vein and/or innominate vein was managed by resection without reconstruction. The SA was dissected with a subadventitial plane on both sides of the tumor then resected. Depending on their involvement, the phrenic nerve and vertebral artery were also resected to achieve tumor-free margins. The preferred SA reconstruction method was direct end-to-end anastomosis, which was often feasible, as the first rib was always removed with the tumor. If the resected arterial segment was too long to allow tension-free end-to-end anastomosis, a ringed polytetrafluoroethylene (PTFE) interposition graft (6 or 8 mm in diameter) was used. The surgical procedure ended with resection of the lung, the remaining chest wall, and spine as dictated by tumor spread. Additional approaches required for extensive resection of the lung and spine were posterolateral thoracotomy (n=18) and a posterior midline incision (n=15), respectively. In patients with carcinoma, the mediastinal lymph nodes were dissected via the same anterior approach. Postoperative anticoagulation therapy was given during the hospital stay and was continued after discharge only in patients with venous resection during 6 months. Lifelong antiplatelet therapy was started during the hospital stay. Finally, duplex ultrasonography was performed before discharge to assess the patency of the reconstructed SA.
Neoadjuvant and adjuvant treatments
Decisions regarding neoadjuvant or adjuvant chemotherapy and radiation therapy were made on a case-by-case basis after discussion with medical oncologists.
Neoadjuvant therapy was given to 34 (40%) patients, 27 with non-small cell lung cancer (NSCLC) and 7 with sarcomas. Of the 27 patients with NSCLC, 16 received platinum-based chemotherapy (n=19) and 8 chemoradiotherapy with a mean radiation dose of 49 Gy (range, 30–60 Gy, n=5). Of the 7 patients with sarcomas, 3 received chemotherapy (adriamycin plus isophosphamide) and 4 chemoradiotherapy with a mean radiation dose of 50 Gy (range, 40–60 Gy).
Adjuvant therapy was given to 54 patients (64%). In the 48 patients with NSCLC, adjuvant therapy consisted mainly of platinum-based chemotherapy with radiation therapy in a mean dose of 48 Gy (range 30 to 65 Gy, n=38) or platinum-based chemotherapy alone (n=10). Of the 5 patients with sarcomas, 3 received chemoradiotherapy and 2 chemotherapy alone.
Operative mortality, survival, and follow-up
Operative mortality was defined as death within the first 30 days after surgery or during the same hospital admission.
Survival was calculated from the date of SA resection to death or last follow-up. Disease-free survival was defined as the time from SA resection to last follow-up or disease recurrence. Complete follow-up was available for all patients.
Follow-up imaging studies consisted of routine chest roentgenograms, chest CT, and duplex ultrasonography of the SA reconstruction. Subclavian arteriography was performed when duplex ultrasonography findings were abnormal.
Graft patency was assessed based on direct color duplex imaging or on evidence of restored blood flow in the ipsilateral arm as manifested by the presence of palpable pulses, absence of pressure gradients, and freedom from clinical symptoms.
Statistical analysis
Data are described as mean with range. Survival and patency rates were calculated using life-table analysis. Kaplan-Meier curves were plotted and compared using the log-rank test for univariate analysis. A multivariate analysis of independent prognostic factors was performed using the Cox proportional hazards stepwise model, with Statview V (Abacus Concept, Berkeley, CA, USA). Differences were considered significant when P was smaller than 0.05.
Results
Surgical resection
All patients underwent complete en bloc resection of the TI malignancy and invaded SA segment, which was on the right side in 45 patients and on the left side in 41 patients. Associated lung resections consisted in lobectomy (n=55), bilobectomy (n=1), pneumonectomy (n=1), or wedge resection (n=19). When additional chest wall resection was performed, the first rib was removed, with one (n=29), two (n=22), or three (n=6) adjacent ribs and/or a portion of the spine (n=13). The lowest root of the brachial plexus (T1) was divided in 46 patients (54%), the C8 root in 2 patients (2%), and the phrenic nerve in 19 patients (22%). The subclavian and brachiocephalic veins were also resected in 65 patients (76%).
Primary end-to-end anastomosis was performed in 48 (56%) patients. PTFE graft interposition was used in 28 (33%) patients with resection of a long arterial segment. Carotid-SA transposition was performed in 8 (10%) patients. To achieve free-margin SA resection, 2 patients required resection of the SA origin under cardiopulmonary bypass with aortic arch repair and SA-to-carotid artery transfer. Finally, SA revascularization was achieved in 1 patient using the superficial femoral artery in an anterolateral thigh flap to cover a skin defect due to radiation-induced sarcoma of the cervicothoracic junction.
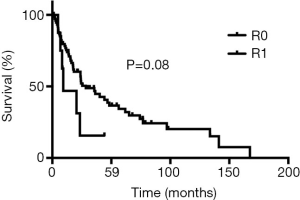
The definite pathological examination showed complete R0 resection in 75 (88%) patients had and R1 resection in 10 (11%) patients. Adjuvant chemoradiotherapy was given to a patient with a positive periarterial margin and to a patient with a positive bone margin and postoperative pneumonia requiring prolonged ventilatory support and therefore precluding repeat surgery. The remaining 7 patients with R1 resection had a positive margin within the brachial plexus and were treated by adjuvant radiation therapy.
Morbidity and operative mortality
There were no postoperative deaths. Inhospital morbidity was 31% and included pneumonia requiring prolonged ventilatory support (n=16), bleeding requiring reoperation (n=3), recurrent-nerve palsy (n=2), empyema requiring surgical debridement (n=1), incision reopening (n=1), cerebrospinal fluid leakage requiring ventriculoperitoneal shunt implantation (n=1), acute pulmonary embolism (n=1), chylothorax (n=1), and peptic ulcer requiring histamine antagonist therapy (n=1). There were no cases of graft infection, graft occlusion, upper limb ischemia, or complications related to SA resection. Mean length of intensive care unit and hospital stays were 8±9 and 21±11 days, respectively.
Long-term follow-up
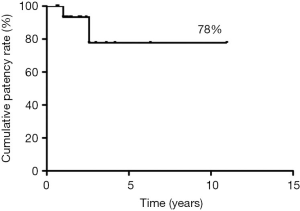
Median follow-up was 44 months, during which 2 patients experienced delayed asymptomatic PTFE graft occlusion, after 12 and 31 months, respectively. The 5-year patency rate was 78% (Figure 2). No cases of occlusion or stenosis were diagnosed in patients with end-to-end SA anastomosis.
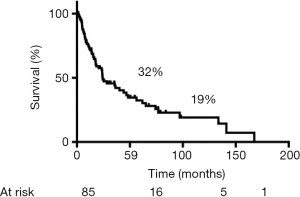
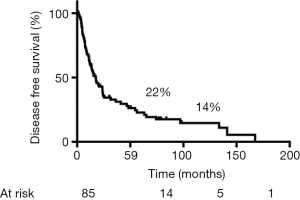
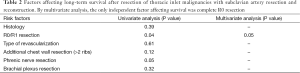
During follow-up, 56 (65%) patients died, 54 of tumor recurrence, 1 of pneumonia, and 1 of multiorgan failure. Overall 5-year and 10-year survival rates were 32% and 19%, respectively (Figure 3), and overall 5-year disease-free survival rate was 22% (Figure 4). Median time to recurrence was 24 months (range, 2 to 168 months). Recurrences were general in 29 (34%) patients, local in 10 (11%) patients, and both in 7 (8%) patients. The local recurrences involved the 4R lymph nodes (n=5), 7 lymphnodes (n=1) or brachial plexus (n=4). Factors affecting long-term survival by univariate analysis are reported Table 2. By multivariate analysis, the only independent factor affecting survival was complete R0 resection (P=0.04; odds ratio, 0.61; 95% confidence interval, 0.25 to 0.97) (Figure 5).
Full table
Discussion
Our study shows that SA resection for TI malignancies through the anterior transcervical approach is safe and can provide long-term survival with low postoperative mortality and a low rate of vascular complications.
We previously reported our initial experience with SA resection through our anterior approach during en bloc NSCLC resection (6). The results were encouraging in terms of safety, reliability, and survival. We therefore use this transcervical approach for all malignancies invading the SA and requiring surgical resection. In 2013, we published the results of our surgical approach for the management of malignancies invading the SA showing that prolonged survival could be achieved in selected patients (9).
The transclavicular approach provides good exposure of the entire TI, allowing safe dissection of the brachial plexus and subclavian vessels (2,3). Most lung resections are feasible through this approach provided retraction of the first ribs is possible. One should know that the extent of the exposure increases with the number of removed ribs. An additional posterolateral approach for major lung resection or a posterior midline incision for spine resection could be used if needed (10). Since TI malignancies invading the SA are located anteriorly, resection of the medial part of the clavicle is preferred over the transmanubrial approach (11) to safely achieve complete cancer resection (1). Interestingly, it has been shown that resection of the inner part of the clavicle doesn’t result in bad cosmetic and functional result provided the posterior cervical nerves (spinal accessory, dorsal scapular, and long thoracic nerves) are preserved (12). The anterior transcervical approach should be the preferred approach when resecting anterior TI malignancies because the posterior approach to the TI cannot provide the same safe exposure of the tumor, brachial plexus, phrenic nerve, subclavian vessels, and vertebral artery as does the anterior one.
SA resection for TI malignancies is a demanding procedure that can provide long-term survival in highly selected patients. Briefly, histological documentation of the malignancy must be obtained. In addition, a complete preoperative workup including FDG-PET and cerebral imaging must be performed to rule out cancer dissemination. In patients with carcinoma, mediastinal lymph node involvement should be ruled out by mediastinoscopy or endobronchial ultrasound biopsy if findings are abnormal by CT or FDG-PET (13,14). As a reminder, T4N2 NSCLC is associated with limited long-term survival even when complete resection is achieved (5). In patients with TI tumors extending up to the neck, the brachial plexus must be assessed by MRI, as involvement higher than the C8 root contraindicates surgical resection. Finally, patients must be evaluated for stenosis of the carotid arteries or ipsilateral vertebral artery, which might result in complications after SA resection.
Ligation of the vertebral artery did not result in neurological complications when no additional cerebrovascular disease was present (15). However, once the tumor and invaded portion of the SA are resected, the SA must be reconstructed. SA ligation without revascularization during resection of TI malignancies consistently causes upper limb ischemia (16,17), as the collateral arterial circulation to the arm is disrupted during tumor dissection. For SA reconstruction, end-to-end or end-to-side anastomosis is preferred over PTFE graft interposition, as the use of prosthetic material carries a risk of bypass occlusion and graft infection. The first rib is always resected, resulting in a more direct trajectory of the SA, which usually allows end-to-end anastomosis of the SA stumps. When the proximal SA stump is too short, end-to-side anastomosis of the SA to the carotid artery is performed. After dissection, the carotid artery is mobilized to the distal SA stump to allow tension-free anastomosis. Thus, a PTFE graft is used only after extensive arterial resection precluding possible anastomosis.
When prosthetic interposition was mandatory, we consistently used PTFE, because many patients received postoperative radiation therapy. Radiation therapy results in localized fibrotic changes, and PTFE grafts are known to have high long-term patency rates within irradiation fields (16,18-21). We preferably use ringed PTFE grafts, which seem particularly resistant to fibrosis (6,22,23). With this strategy, the overall 5-year patency rate was 78%, and only 2 patients had PTFE graft occlusion. In both cases, graft occlusion was delayed and asymptomatic. Upper limb angiography showed extensive collateral circulation from the external carotid and internal thoracic arteries to the arm, explaining the absence of clinical upper-limb ischemia. None of the patients with reconstruction by direct anastomosis experienced occlusion or stenosis. Despite the use of radiation therapy in many of our patients, the SA reconstruction outcomes were similar to those reported after SA revascularization for occlusion by atheroma plaque (19-22,24-27).
SA invasion has been reported to be an independent risk factor for decreased long-term survival (5,6,10,27,28). The thick arterial wall with a solid adventitial layer usually constitutes an effective barrier to local cancer spread. Thus, arterial invasion indicates a highly aggressive tumor. However, we have obtained long-term survival after complete R0 resection without any additional morbidity and mortality compared to TI malignancies without SA invasion. In earlier studies, overall 5-year survival after resection of TI malignancies ranged from 18% to 56% (4,8,29-36). Therefore, the 5-year survival of 32% in our update indicates that resection of TI malignancies invading the SA is a valid treatment option in selected patients, provided complete R0 resection can be achieved. R0 resection was the only factor that independently affected long-term survival in our study. Surgery can also achieve good local control of the disease, avoiding severe pain due to tumor spread through the brachial plexus. Few patients experienced local recurrences at the neck at the level of the brachial plexus, and no recurrences were noted at the level of the SA resection. This finding highlights the importance of brachial plexus MRI during the preoperative workup to rule out tumor spread above the C8 root.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- de Perrot M, Rampersaud R. Surgical approaches to apical thoracic malignancies. J Thorac Cardiovasc Surg 2012;144:72-80. [PubMed]
- Fischer S, Darling G, Pierre AF, et al. Induction chemoradiation therapy followed by surgical resection for non-small cell lung cancer (NSCLC) invading the thoracic inlet. Eur J Cardiothorac Surg 2008;33:1129-34. [PubMed]
- Wright CD, Menard MT, Wain JC, et al. Induction chemoradiation compared with induction radiation for lung cancer involving the superior sulcus. Ann Thorac Surg 2002;73:1541-4. [PubMed]
- Dartevelle PG, Chapelier AR, Macchiarini P, et al. Anterior transcervical-thoracic approach for radical resection of lung tumors invading the thoracic inlet. J Thorac Cardiovasc Surg 1993;105:1025-34. [PubMed]
- Yildizeli B, Dartevelle PG, Fadel E, et al. Results of primary surgery with t4 non-small cell lung cancer during a 25-year period in a single center: The benefit is worth the risk. Ann Thorac Surg 2008;86:1065-75; discussion 1074-5. [PubMed]
- Fadel E, Chapelier A, Bacha E, et al. Subclavian artery resection and reconstruction for thoracic inlet cancers. J Vasc Surg 1999;29:581-8. [PubMed]
- Kunitoh H, Kato H, Tsuboi M, et al. A randomised phase II trial of preoperative chemotherapy of cisplatin-docetaxel or docetaxel alone for clinical stage IB/II non-small-cell lung cancer results of a Japan Clinical Oncology Group trial (JCOG 0204). Br J Cancer 2008;99:852-7. [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: Long-term results of southwest oncology group trial 9416 (intergroup trial 0160). J Clin Oncol 2007;25:313-8. [PubMed]
- Lahon B, Mercier O, Fadel E, et al. Subclavian artery resection and reconstruction for thoracic inlet cancer: 25 years of experience. Ann Thorac Surg 2013;96:983-8; discussion 988-9. [PubMed]
- Fadel E, Missenard G, Court C, et al. Long-term outcomes of en bloc resection of non-small cell lung cancer invading the thoracic inlet and spine. Ann Thorac Surg 2011;92:1024-30; discussion 1030. [PubMed]
- Grünenwald D, Spaggiari L. Transmanubrial osteomuscular sparing approach for apical chest tumors. Ann Thorac Surg 1997;63:563-6. [PubMed]
- De Perrot M, Rampersaud R. Anterior transclavicular approach to malignant tumors of the thoracic inlet: Importance of the scapulothoracic articulation. J Thorac Cardiovasc Surg 2007;134:801-3. [PubMed]
- Webb WR, Jeffrey RB, Godwin JD. Thoracic computed tomography in superior sulcus tumors. J Comput Assist Tomogr 1981;5:361-5. [PubMed]
- O’Connell RS, McLoud TC, Wilkins EW. Superior sulcus tumor: Radiographic diagnosis and workup. AJR Am J Roentgenol 1983;140:25-30. [PubMed]
- Watanabe T, Okada Y, Sakurada A, et al. Resection of apical lung carcinoma involving the vertebral artery. Ann Thorac Surg 2010;90:302-3. [PubMed]
- Paulson DL. Carcinomas in the superior pulmonary sulcus. J Thorac Cardiovasc Surg 1975;70:1095-104. [PubMed]
- Wright CD, Mathisen DJ. Superior sulcus tumors. Curr Treat Options Oncol 2001;2:43-9. [PubMed]
- Dartevelle PG, Chapelier AR, Pastorino U, et al. Long-term follow-up after prosthetic replacement of the superior vena cava combined with resection of mediastinal-pulmonary malignant tumors. J Thorac Cardiovasc Surg 1991;102:259-65. [PubMed]
- AbuRahma AF, Robinson PA, Khan MZ, et al. Brachiocephalic revascularization: A comparison between carotid-subclavian artery bypass and axilloaxillary artery bypass. Surgery 1992;112:84-91. [PubMed]
- Rosenthal D, Ellison RG Jr, Clark MD, et al. Axilloaxillary bypass: Is it worthwhile? J Cardiovasc Surg (Torino) 1988;29:191-5. [PubMed]
- Schanzer H, Chung-Loy H, Kotok M, et al. Evaluation of axillo-axillary artery bypass for the treatment of subclavian or innominate artery occlusive disease. J Cardiovasc Surg (Torino) 1987;28:258-61. [PubMed]
- AbuRahma AF, Robinson PA, Jennings TG. Carotid-subclavian bypass grafting with polytetrafluoroethylene grafts for symptomatic subclavian artery stenosis or occlusion: A 20-year experience. J Vasc Surg 2000;32:411-8; discussion 418-9. [PubMed]
- Morisaki Y, Takagi K, Furuya T, et al. Complete resection of left apical invading lung cancer, following reconstruction of subclavian artery by artificial graft--a case report. Nihon Kyobu Geka Gakkai Zasshi 1993;41:1074-8. [PubMed]
- Criado FJ, Queral LA. Carotid-axillary artery bypass: A ten-year experience. J Vasc Surg 1995;22:717-22; discussion 722. [PubMed]
- Perler BA, Williams GM. Carotid-subclavian bypass--a decade of experience. J Vasc Surg 1990;12:716-22; discussion 722. [PubMed]
- Vitti MJ, Thompson BW, Read RC, et al. Carotid-subclavian bypass: A twenty-two-year experience. J Vasc Surg 1994;20:411-7; discussion 417-8. [PubMed]
- Sterpetti AV, Schultz RD, Feldhaus RJ, et al. Seven-year experience with polytetrafluoroethylene as above-knee femoropopliteal bypass graft. Is is worthwhile to preserve the autologous saphenous vein? J Vasc Surg 1985;2:907-12. [PubMed]
- Fadel E, Missenard G, Chapelier A, et al. En bloc resection of non-small cell lung cancer invading the thoracic inlet and intervertebral foramina. J Thorac Cardiovasc Surg 2002;123:676-85. [PubMed]
- Fukuse T, Wada H, Hitomi S. Extended operation for non-small cell lung cancer invading great vessels and left atrium. Eur J Cardiothorac Surg 1997;11:664-9. [PubMed]
- Okubo K, Wada H, Fukuse T, et al. Treatment of pancoast tumors. Combined irradiation and radical resection. Thorac Cardiovasc Surg 1995;43:284-6. [PubMed]
- Alifano M, D’Aiuto M, Magdeleinat P, et al. Surgical treatment of superior sulcus tumors: Results and prognostic factors. Chest 2003;124:996-1003. [PubMed]
- Carpenter SG, Stone WM, Bower TC, et al. Surgical management of tumors invading the aorta and major arterial structures. Ann Vasc Surg 2011;25:1026-35. [PubMed]
- Goldberg M, Gupta D, Sasson AR, et al. The surgical management of superior sulcus tumors: A retrospective review with long-term follow-up. Ann Thorac Surg 2005;79:1174-9. [PubMed]
- Hagan MP, Choi NC, Mathisen DJ, et al. Superior sulcus lung tumors: Impact of local control on survival. J Thorac Cardiovasc Surg 1999;117:1086-94. [PubMed]
- Martinod E, D’Audiffret A, Thomas P, et al. Management of superior sulcus tumors: Experience with 139 cases treated by surgical resection. Ann Thorac Surg 2002;73:1534-9; discussion 1539-40. [PubMed]
- Wright CD, Moncure AC, Shepard JA, et al. Superior sulcus lung tumors. Results of combined treatment (irradiation and radical resection). J Thorac Cardiovasc Surg 1987;94:69-74. [PubMed]



