Combined treatment modalities in Pancoast tumor: results of a monocentric retrospective study
Introduction
Pancoast tumor, as otherwise known superior sulcus tumor (SST), was described for the first time by the radiologist Henry K. Pancoast in 1924 (1). He mistakenly believed that these tumors emanated from epithelial rest cells of the last branchial cleft. In 1932, J. Tobias recognized their site of origin as bronchopulmonary tissue and his name is associated (2). SST was defined with a lot of criteria of neoplasia involvement: brachial plexus, endothoracic fascia, subclavian vessels, vertebral bodies, first, second and third ribs. Actually with new technology of imaging, as magnetic resonance imaging (MRI) (3) these tumors are smaller at the diagnostic so they do not gather all the criteria. Today, to be called Pancoast tumor, an apical lung tumor (4) must invade the parietal pleura and thoracic wall that causes pain, paresthesia or other neurological dysfunction as Horner’s syndrome (4).
Pancoast stated that these tumors were “not subject to surgical removal”, “refractory to radiation treatment”, and so “rapidly fatal” (1) and prior to 1950’s, these tumors were evenly fatal. The first successful treatment by surgery and postoperative radiotherapy was reported by Chardack and Maccallum in 1956 (5). In 1961, Shaw and Paulson published the first series of 18 patients treated by preoperative irradiation (30 to 35 Gy with cobalt 60) and surgery (6). Since that time, different treatments that include chemotherapy, radiation and surgery, alone or in combination have been used. Chemotherapy as a single modality has historically been reserved for palliation in patients with metastatic disease. Compared with the results of radio-surgical therapy, modern or high dose exclusive radiation therapy have clearly demonstrated its inferiority for tumors which are resectable (7). Moreover most retrospectives studies using radiotherapy alone cannot be adequately evaluated because staging, dose variations, lack of reporting of treatment-related morbidity and radiation modalities were not well reported. Since the last decade, novel technique of radiation and surgery have been developed, however the most significant advancement in the treatment of SST has been the addition of chemotherapy to the induction radiotherapy (2,8,9). Actually chemoradiotherapy (CRT) followed by extensive en-bloc resection is one standard of care for any potentially resectable SST with better local control and overall survival (OS) at 5 years of 44% to 59% (9-11).
In an attempt to identify prognostic factor, this article reviews the data from our own institution in terms of number of complete response, modality of relapse, side-effects and mortality in a consecutive series of SST patients treated with an induction CRT.
Patients and methods
Eligibility criteria
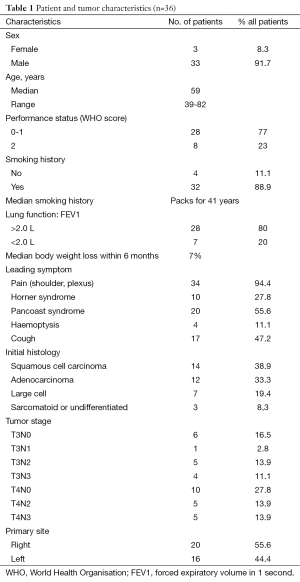
We retrospectively analyzed all patients with histologically confirmed primitive non-small cell lung cancer (NSCLC) of the superior sulcus with clinical stage T3 or T4 treated with the same multimodality approach in our institution from 1992 to 2005. Preoperative pathologic diagnosis was made with computed tomography CT-guided fine-needle aspiration biopsy, bronchial biopsy per endoscopy or mediastinoscopy. A complete staging included chest-abdominal CT, brain CT or MRI, positron emission tomography (PET) when it was possible and isotopic bone scan if needed.
Other eligibility criteria included a World Health Organisation (WHO) performance status of 0-2, no prior treatment for lung cancer or other concurrent malignancy, percentage of weight loss less than 10%.
Characteristics that rendered patients ineligible or functionally inoperable were: a predicted postoperative forced expiratory volume in 1 second (FEV1) <0.8 L, cardiac dysfunction, significant carotid stenosis detected with supra aortic ultrasonography.
All patients were required to have an adequate hematologic, renal, hepatic function.
Treatment modalities
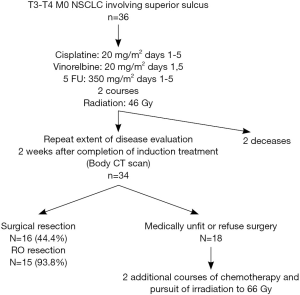
The treatment algorithm for these patients is shown in Figure 1.
Induction chemotherapy
Patients received two courses of cisplatin-vinorelbine-5 fluorouracil (5FU) with a 3-week interval. Cisplatin was administered on day 1 to 5 at 20 mg/m2 with hydration and antiemetics, vinorelbine on day 1-5 at 20 mg/m2 and 5FU continue from day 1 to 5 at 350 mg/m2. When creatinine values rose above 60 mL/min cisplatin was replaced by carboplatin AUC 5 and treatment was continued. The second course of chemotherapy was started when the toxicities recovered to grade 1 or 0.
Induction radiotherapy
Three-dimensional (3D) conformal thoracic radiation was started on day 1 of chemotherapy with a linear accelerator. The planning target volume contained the primary tumor with a margin of 13 to 15 mm enclosing the ipsilateral supraclavicular region, the ipsilateral hilum, the ipsilateral mediastinal. Radiation portals were defined by means of 3D CT scan reconstruction to minimize toxicity to nearby structures, as spinal cord, brachial plexus and esophagus. The first time of radiation was performed to deliver a mean dose of 44-45 Gy with daily 1.8 to 2 Gy in 22 or 25 fractions over 6 weeks.
Surgery
Two or three weeks after the completion of CRT, complete restaging, without mediastinoscopy, was performed with discussion in a multidisciplinary staff. If possible, surgery followed, 4-6 weeks after radiation. The standard surgical technique to access the upper thoracic was Paulson approach or anterior type (Masaoka), the specific surgical technique was selected on the basis of the location and local involvement of the primary tumor. At the time of the operation, a lobectomy or pneumonectomy with chest wall resection was performed. Lesser pulmonary resections were not used. Areas of direct tumor extension into the spine were resected en bloc with the involved lung with a neurosurgical operative assistance if necessary. In all cases, a systematic lymphadenectomy of the interlobar, hilar and ipsilateral mediastinal lymph node stations was performed. Routine coverage of the bronchial stump with omentum flap was used.
Boost therapy
For unresected tumor after induction CRT, boost radiotherapy up to 66 Gy was done with a concomitant third course of chemotherapy.
Response and toxicity evaluation
Acute side-effects of CRT were evaluated according to the National Cancer Institute Common Toxicity Criteria scale version 2.0 (NCI CTC v2.0). Responses to treatment were assessed using standard WHO criteria (12).
Surgery perioperative morbidity and mortality were prospectively collected and recorded in the EPITHOR data base of the Société Française de Chirurgie Cardio-Vasculaire et Thoracique (SFCVT). Surgical resection was defined as complete when the following criteria were satisfied: a pathological complete response (pCR) was demonstrated when the resected surgical specimen contained no evidence of viable cancer cells. A resection was considered R0 when no viable tumor was remaining in the operative field, whereas an R1 resection left only microscopic tumor foci.
After completion of multimodality treatment, patients were evaluated every 3 months during the first 2 years postoperative and every 6 months after on the basis of history.
Statistical analysis
Statistical analysis was performed using the SEM software (13). Standard tests were used (χ2 test, analysis of variance, Student’s t-test) to study the relationships between the parameters. Non-parametric tests were chosen whenever distributions were not Gaussian or when variances differed (Mann-Whitney test, Kruskal-Wallis test, Spearman’s rank correlation). Survival analysis was performed using the Kaplan-Meier method and the curves were compared using the log-rank test. Multivariate analyses on tumor response were conducted using logistic regression and Cox regression model on survival. P values <0.05 were considered significant.
Results
Patient characteristics
From 1992 to 2005, 36 consecutive patients were treated in our institution with the same modality and retained for this study. The patients’ characteristics were showed in Table 1 and the treatment schedule was presented in Figure 1. Thirty-six cases were analyzed to determine toxicities, response rates, surgical and pathologic results, PFS, and OS.
Full table
Induction chemoradiation
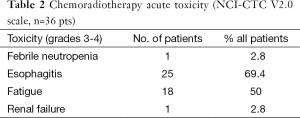
The induction therapy was completed in 34 (94.4%) on the 36 patients. In two cases, only one course of chemotherapy was dispensed because of 1 toxic death on day 5 and 1 gastrointestinal perforation-resulting from respectively primary severe myelosuppression and secondary sepsis shock. Table 2 lists the other major side-effects of the protocol therapy.
Full table
Clinical response to induction treatment
In 34 patients, the tumor response to induction CRT was assessed on CT scan with RECIST criteria (14). The responses were: 12 partial responses (PR) (35.3%); 12 stable diseases (SD) (35.3%); 6 progression diseases (PD) (17.6%), not assessable, 4 patients. Pain was relieved for 92%.
Surgery
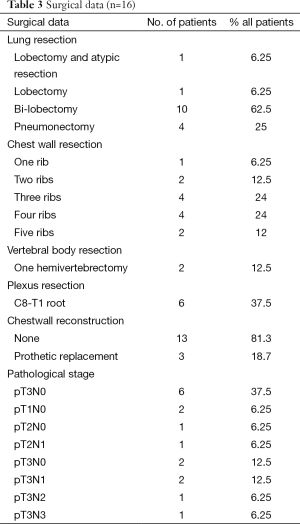
Thoracotomy was performed in 16 (47%) of the 34 patients who received the induction CRT. The reasons for non-operability were: inadequate lung function for 3 patients, too worse WHO criteria for 8 patients, tumor not completely resecable for 6 patients and 1 patient refusal. The surgical procedures were performed as follows: 62.5% lobectomy, 25% pneumonectomy, one patient needed a bilobectomy and another one had a lobectomy and atypic resection. Details of surgical procedure are mentioned in Table 3. The results of surgery were as follows: 93.8% of complete surgical resection (RO), 43.8% of pathologically complete response with 5 years OS of 57.1% in this group. Significant morbidities occurred in 9 (56.3%) patients without dominant pattern (Table 4). The major postoperative morbidity was infection with sternite, pneumonia, pleural effusion, cerebrospinal fluid break on vertebral resection. One death occurred in the perioperative period because of respiratory failure with a lot of complication due to long course in reanimation.
Full table
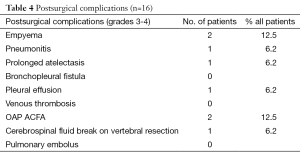
Full table
Boost chemoradiotherapy (CRT)
For the 18 patients with non-operable disease after the induction CRT, treatment was completed with boost radiotherapy of 22 Gy in 11 fractions and concomitant third cycle of chemotherapy. The final response rate for these patients was: 4 patients (22%) with stabilization, 5 (27%) with PR, 7 (39%) with progression, 2 non assessable.
Disease-free survival (DFS) and OS
Figures 2 and 3 show the DFS and OS curves in different groups, updated in Mars 2015.
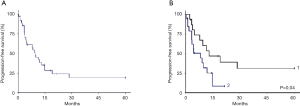
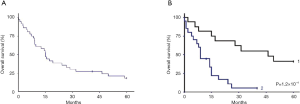
The median of follow-up period was 38.6 months [2-206]. In the operated group, the median DFS time was 12.9 months with DFS rates at 1 and 2 years 53.6% and 39.1% respectively. The median OS was 46.4 months. The OS rates at 2 and 5 years were 68.8% and 37.5% respectively. Five-year survival was 57% for patients with resected disease and complete pathologic response.
In the unresectable group, the median DFS time was 8.1 months. The DFS rate at 1 year was 25.2%. The median OS was 9.1 months. The OS rates at 1 and 2 years were 45% and 16.9% respectively.
Prognostic factors were: (I) the clinical response to the induction CRT; (II) the possibility of surgery or not (Table 5, multivariate analysis); and (III) the pCR or not (P=0.039, univariate analysis).

Full table
Relapse
Recurrences were found in 26 (72%) of patients. Brain metastasis was the most common site of recurrence (n=9), followed by other distant recurrences (n=10) (lung, bone, liver, surrenal) and local relapses (n=7). Eighteen patients (72%) have been treated at the relapse with a second line of chemotherapy and brain irradiation in five cases of brain metastasis.
Discussion
The promising results of combined CRT and surgery used in stage IIIA/B NSCLC have been the rationale to test this modality in patients with SST (15). SSTs represent 3-5% of all NSCLC located in the lung apex (16). Previous studies showed a high local resectability rate and increased long-term survival for SST patients treated with combined modalities (9,17,18) and locally advanced tumors with invasion of chest wall, and adjacent structures, render the primary surgery less efficient.
In locally advanced inoperable NSCLC, chemotherapy increases the efficacy of radiation (19) and randomized studies comparing concurrent vs. sequential CRT showed that the concurrent approach provided a superior outcome (20). This better efficiency of concurrent chemoradiation was due to supra-additive effects of drugs and radiation but also to the development of 3D conformal radiotherapy better sparing healthy tissues in the vicinity of clinical tumor volume (CTV).
In our study, 36 patients T3-4, N0-3 NSCLC received two courses of cisplatin/vinorelbine and fluorouracil concurrently with radiation (44 Gy) with 71% of objective response (OR), comparable to published data (21). Grade 3-4 toxicity was observed in 69% of cases especially esophagitis. There were only six cases of grade 4 esophagitis who required parenteral fluid nutrition and no death. This high rate was probably due to the use of 5FU. At the end of the CRT the mean weight loss was 3 kg for all the population.
After this induction CRT, the resection was feasible and associated with high rate of completeness surgically RO resection (93.8%). The complete pathologically response was achieved in 44% of patients compared very favorably to previously studies who reported a pCR from 16% to 40% (16,21-23). The postoperative mortality rate (0-3 months) was acceptable in patients who received lobectomy (75%). In our study, a routine coverage of the bronchial stump with omentum flap was used to reduce morbidity and mortality of surgery process (24). We did not observe any bronchopulmonary operative complication within irradiated volume.
No prognostic difference between T3 and T4 tumors was observed in agreement with other studies (9). Five-year survival was 57% for patients with resectable disease and complete pathologic response. Pathologic CR led to better survival than when any residual disease was present (five-year survival: 28.5% in this case). Disease progression occurred mainly in distant sites.
Here, 19 (53%) patients with N2-N3, known to have the poorest prognosis (25) were included, though they were excluded from both SWOG and JCOG trials (9,26).There were no statistical difference in OS or DFS according to nodal status because N3 ipsilateral supraclavicular nodes involvement seemed to behave like N1 nodes as in the study of Kwong et al. (23). At this time, in Pancoast tumor, N3 ipsilateral supraclavicular nodes located in the vicinity of the lesion must be considerate as local nodes with a behavior prognostic significance similar to that of N1 disease. Only N2 tumor diseases have clearly demonstrated a worse outcome than those with N0 or N1 diseases. In our study all N2 disease (nine cases) have relapse within 2 years.
A third drug, 5FU, was added to standard doublet of cisplatine/vinorelbine in order to increase radiosensitization but the local tumor control was as observed in other trials, only the pCR was higher (27). Nevertheless this association increases toxicity especially esophagitis.
At the moment, the main challenge is to find a new regimen that can be associated with radiation in order to increase the radiosensitivity but not the toxicity and decrease the occurrence of distant relapse. Furthermore, the ability to deliver adjuvant chemotherapy after CRT and surgery is poor because patients with Pancoast tumor may not tolerate more extensive treatment. So new drugs, like pemetrexed or targeted therapies are promising (28,29) considering that most of Pancoast tumors are non-squamous carcinomas but they rarity compromise prospective large studies.
While retrospective and including a limited series of patients, this study was homogeneous regarding treatment modalities and taking care of consecutive patients in a single institution.
In conclusion, our study confirms a beneficial impact of induction CRT prior surgery in SST patients. Notwithstanding some increased esophagitis, this trimodality treatment regimen was well tolerated and conferred a high rate of local control. Ipsilateral supraclavicular N3 patients should be included in this trimodality treatment. High occurrence of brain metastasis suggests the possibility of prophylactic cranial irradiation (PCI) (11). However the RTOG 0214 study, evaluating the impact of PCI on patients with locally advanced lung cancer, had revealed that despite the decrease of brain relapse there was no significant impact on survival (30). On this basis, at the present time, there is no evidence to support the routine used of PCI in Pancoast tumours (29).
Further studies with new induction drug regimen (including targeted and immunological therapies) combined with 3D-conformal RT are needed to improve local control while reducing (brain) metastasis and OS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pancoast HK. Importance of careful roentgen-ray investigations of apical chest tumors. JAMA 1924;83:1407-11.
- Arcasoy SM, Jett JR. Superior pulmonary sulcus tumors and Pancoast's syndrome. N Engl J Med 1997;337:1370-6. [PubMed]
- Manenti G, Raguso M, D'Onofrio S, et al. Pancoast tumor: the role of magnetic resonance imaging. Case Rep Radiol 2013;2013:479120.
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6 Suppl 1:S108-15. [PubMed]
- Chardack WM, Maccallum JD. Pancoast tumor; five-year survival without recurrence or metastases following radical resection and postoperative irradiation. J Thorac Surg 1956;31:535-42. [PubMed]
- Shaw RR, Paulson DL, Kee JL. Treatment of Superior Sulcus Tumor by Irradiation Followed by Resection. Ann Surg 1961;154:29-40. [PubMed]
- De Bari B, Lestrade L, Souquet PJ, et al. Single French centre retrospective analysis of local control after high dose radiotherapy with or without chemotherapy and local control for Pancoast tumours. Cancer Radiother 2012;16:107-14. [PubMed]
- Shahian DM, Neptune WB, Ellis FH Jr. Pancoast tumors: improved survival with preoperative and postoperative radiotherapy. Ann Thorac Surg 1987;43:32-8. [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: Initial results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Thorac Cardiovasc Surg 2001;121:472-83. [PubMed]
- Ginsberg RJ, Martini N, Zaman M, et al. Influence of surgical resection and brachytherapy in the management of superior sulcus tumor. Ann Thorac Surg 1994;57:1440-5. [PubMed]
- Tamura M, Hoda MA, Klepetko W. Current treatment paradigms of superior sulcus tumours. Eur J Cardiothorac Surg 2009;36:747-53. [PubMed]
- Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981;47:207-14. [PubMed]
- Kwiatkowski F, Girard M, Hacene K, et al. Sem: a suitable statistical software adaptated for research in oncology. Bull Cancer 2000;87:715-21. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- Eberhardt W, Wilke H, Stamatis G, et al. Preoperative chemotherapy followed by concurrent chemoradiation therapy based on hyperfractionated accelerated radiotherapy and definitive surgery in locally advanced non-small-cell lung cancer: mature results of a phase II trial. J Clin Oncol 1998;16:622-34. [PubMed]
- Blaauwgeers JL, Kappers I, Klomp HM, et al. Complete pathological response is predictive for clinical outcome after tri-modality therapy for carcinomas of the superior pulmonary sulcus. Virchows Arch 2013;462:547-56. [PubMed]
- Wright CD, Menard MT, Wain JC, et al. Induction chemoradiation compared with induction radiation for lung cancer involving the superior sulcus. Ann Thorac Surg 2002;73:1541-4. [PubMed]
- Marra A, Eberhardt W, Pottgen C, et al. Induction chemotherapy, concurrent chemoradiation and surgery for Pancoast tumour. Eur Respir J 2007;29:117-26. [PubMed]
- Jassem J. The role of radiotherapy in lung cancer: where is the evidence? Radiother Oncol 2007;83:203-13. [PubMed]
- Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17:2692-9. [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313-8. [PubMed]
- Pourel N, Santelmo N, Naafa N, et al. Concurrent cisplatin/etoposide plus 3D-conformal radiotherapy followed by surgery for stage IIB (superior sulcus T3N0)/III non-small cell lung cancer yields a high rate of pathological complete response. Eur J Cardiothorac Surg 2008;33:829-36. [PubMed]
- Kwong KF, Edelman MJ, Suntharalingam M, et al. High-dose radiotherapy in trimodality treatment of Pancoast tumors results in high pathologic complete response rates and excellent long-term survival. J Thorac Cardiovasc Surg 2005;129:1250-7. [PubMed]
- Stamatis G, Djuric D, Eberhardt W, et al. Postoperative morbidity and mortality after induction chemoradiotherapy for locally advanced lung cancer: an analysis of 350 operated patients. Eur J Cardiothorac Surg 2002;22:292-7. [PubMed]
- Rusch VW, Parekh KR, Leon L, et al. Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J Thorac Cardiovasc Surg 2000;119:1147-53. [PubMed]
- Kunitoh H, Kato H, Tsuboi M, et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group trial 9806. J Clin Oncol 2008;26:644-9. [PubMed]
- Segawa Y, Ueoka H, Kiura K, et al. A phase II study of cisplatin and 5-fluorouracil with concurrent hyperfractionated thoracic radiation for locally advanced non-small-cell lung cancer: a preliminary report from the Okayama Lung Cancer Study Group. Br J Cancer 2000;82:104-11. [PubMed]
- Girard N, Mornex F. Radiotherapy and targeted therapies in non-small-cell lung cancer. Bull Cancer 2009;96:311-9. [PubMed]
- Nuijten M, Heigener DF, Bischoff HG, et al. Effectiveness of bevacizumab- and pemetrexed-cisplatin treatment for patients with advanced non-squamous non-small cell lung cancer. Lung Cancer 2010;69 Suppl 1:S4-10. [PubMed]
- Gore EM, Bae K, Wong SJ, et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of radiation therapy oncology group study RTOG 0214. J Clin Oncol 2011;29:272-8. [PubMed]