Lung-MAP—framework, overview, and design principles
Introduction
The expanding application of genomic sequencing in oncology is revealing targetable genetic aberrations including gene mutations, rearrangements, amplifications, and deletions, and creating an immense opportunity to implement personalized therapy with a high potential to improve patients outcomes.
In lung adenocarcinoma, potentially actionable genomic alterations are present in approximately 64% of patients (1). The activity of EGFR and ALK inhibitors in patients harboring respectively EGFR activating mutations (~17%) or ALK translocations (4-8%) has changed the treatment paradigm and significantly improved survival of lung adenocarcinoma patients (2,3). New gene aberrations such as ROS1 rearrangements, BRAF mutation, ERBB2 mutation, and RET fusions, are being targeted in clinical trials, with promising preliminary results (1,4-7). Unfortunately, in spite of squamous cell carcinoma (SCC) being the second most common lung cancer histology, corresponding to approximately 25% of cases, no molecular targeted agents have been developed for patients with this histology thus far.
The Cancer Genome Atlas and other sequencing initiatives shed some light into the genomics of lung SCC and revealed potentially targetable alterations such as FGFR mutations, fusions, and amplifications; PI3KCA mutations; CCND1 amplification; and c-MET amplification and/or protein overexpression (8,9). Successful targeting of driver oncogenes in SCC, mirroring lung adenocarcinoma, could lead to a significant improvement in patient’s clinical outcomes and its investigation should be intensively pursued.
The traditional development of oncology therapeutic agents is overall a lengthy, expensive, and inefficient process. The time from initial drug discovery to clinical testing and regulatory review can take up to 15 years. The many challenges of this process include difficulties in the recruitment of patients; high number of screen failures, particularly for trials studying a rare biomarker defined subgroup; the bureaucratic process; cost; and lengthy regulatory review (10). Only 3-5% of adult cancer patients enroll in clinical trials in the United States, leading to underpowered studies, early trial discontinuation, lack of feasibility, and inapplicability of trial results in an evolving medical field (11).
In order to overcome the above-mentioned barriers of cancer drug development and ask more questions in a single study, new approaches to clinical trial designs are being explored. For example, BATTLE (Biomarker-integrated Approaches of Targeted Therapy for Lung cancer Elimination) and I-SPY2 TRIAL (Investigation of Serial studies to Predict Your Therapeutic Response with Imaging And moLecular analysis 2) were pilot studies using adaptive designs combined with biomarker testing. BATTLE accrued refractory non-small cell lung cancer, and based on the results of 11 biomarkers tested in a mandatory fresh biopsy, it adaptively randomized patients to 4 different arms, according to the data obtained during the trial course (12). I-SPY2 is investigating the addition of targeted therapy to chemotherapy in the breast cancer neoadjuvant setting, based on tissue and image biomarkers. It has a master structure that allows up to 5 drugs to be tested simultaneously in independent phase II trials (13).
In order to fulfill the unmet need of lung SCC, the LUNG-MAP (SWOG S1400) protocol was conceptualized by SWOG with the collaboration of public and private groups including the National Cancer Institute, the Food and Drug Administration (FDA), the National Clinical Trials Network, pharmaceutical industry partners, and advocacy organizations. LUNG-MAP is an “umbrella” trial design that facilitates the evaluation of multiple investigational therapies in independently conducted therapeutic studies under a single trial infrastructure in lung SCC population subsets. The goal of this approach is to improve genomic screening and time lines for drug-biomarker testing with the “one-stop-shop” approach allowing for inclusion of the maximum numbers of otherwise eligible patients in comparison with the usually employed “single screen-single trial”. This manuscript will discuss the overall protocol and trial design principles.
Lung-MAP framework and overview
Protocol design
Lung-MAP (S1400) is aiming to identify biomarker-drug pairs that will lead to successful therapeutic outcomes and registration of new agents. It is a registration-intent master protocol that includes a screening and a clinical trial component. The clinical trial component includes multiple sub-studies that independently evaluate investigational therapies. It is designed to be modular such that new sub-studies can be added either as other sub-studies close or as new biomarker-drug pairs are identified for testing in the SCC patient population.
Original eligible population
The eligible population consists of adult patients with recurrent or metastatic lung SCC who progressed after first line platinum based chemotherapy and have a performance status ECOG ≤2. Measurable disease and adequate organ function is required. The presence of an EGFR activating mutation or EML4-ALK translocation as defined by the central screening next generation sequencing performed by Foundation Medicine, Inc. (FMI), uncommon in SCC, is an exclusion criterion.
Screening overview
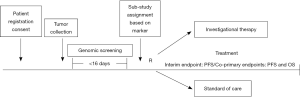
Patient tumor specimens are to be submitted for central testing within 1 day after registration to the screening portion of the trial. Formalin-fixed and paraffin-embedded tissue from archival and/or fresh tumor biopsy must be available for biomarker testing. Submission of either a tumor block or minimum of 12 unstained slides is required for study entry. FMI is performing the biomarker analysis using massive parallel DNA sequencing to detect potentially targetable genomic alterations in cancer related genes. Immunohistochemical assays can also be performed according to the biomarker being investigated. The tests are executed in CLIA (Clinical Laboratory Improvement Amendments) certified laboratories. The turn-around time from tissue submission to reporting of the results is less than or equal to 16 days. Upon completion of biomarker screening, the results are reported to the SWOG statistical center.
Clinical trial component
Within one day of receipt of the tumor profiling results, patients are assigned to a sub-study within the Lung-MAP umbrella. Patients with exactly one of the targeted biomarkers are assigned to the associated sub-study evaluating an investigational therapy targeted against that aberration. For patients with more than one of the targeted biomarkers, assignment is randomized between the sub-studies they are eligible for using an algorithm that gives more weight to studies with lower prevalence biomarkers. Patients whose tumors alterations don’t fall into any of the available matched drug-biomarker sub-studies are assigned to a “non-match” sub-study. Therefore all screened patients who satisfy the clinical eligibility criteria have a study in which to enroll. Figure 1 shows an overview of the study schema. The specific drugs that were included in the original protocol launched in June 2014 are listed in Table 1. Docetaxel is considered the standard of care comparison arm for all sub-studies, except for S1400E, where the comparison drug was Erlotinib.

Full table
Statistical design
Each of the sub-studies are independently conducted, analyzed and reported. The initial statistical design and the primary design of each of the sub-studies is a seamless phase II/III design (16). The sub-studies employ co-primary objectives: (I) to compare overall survival between the investigational therapy arm and the standard of care arm; and (II) to determine if there is both a statistical and clinically-meaningful difference in progression-free survival (PFS) between the treatment arms on the study. “Clinically meaningful” is defined as at least a 2.25 month difference in median PFS between the two arms for sub-studies evaluating single agent targeted therapy/non-match therapy; and at least a 2.5 months difference for sub-studies evaluating targeted therapy or non-match therapy combinations. The expected median PFS and OS for patients receiving standard of care treatment is 3 and 8 months respectively.
The phase II is an interim analysis, which evaluate early stopping due to futility, based on PFS. As such, all patients included in the phase II component of the study are included in the phase III analyses. The ‘bar’ for continuing past the phase II interim analysis is based on phase II design properties and has a much higher chance of stopping the trial than standard phase III interim analyses. This design facilitates both speedy screening of less effective investigational therapies (and thereby closing sub-studies at the phase II interim analysis point which is approximately 100 patient accruals), and accelerates completion of accrual to a phase III and time to a definitive answer for effective investigational therapies. Secondary objectives include a comparison of response rate and toxicity between the arms within a sub-study.
The sample size for each sub-study is determined based on the biomarker prevalence, maintaining all other design parameters the same across sub-studies. It ranges from 68 to 124 patients for a biomarker prevalence of 2.5% to 20%. For the phase III analysis with similar prevalence assumptions, the sample size ranges from 272 to 336. The expected accrual rate in the Master Protocol is 500 to 1,000 patients per year in approximately 400 sites.
Lung-MAP design principles
One of the major principles of Lung-MAP is the rapid implementation of new research findings within the protocol framework and study design. In other words, new sub-studies can enter the trial at any time when relevant drug-biomarker pairs with sufficient proof-of concept become available.
The agent selection for the protocol is the task of a Drug/Biomarker Selection Committee comprised of independent members from the pharmaceutical industry, academia, the Investigational Drug Branch of the NCI, and the lead principal investigators. A formal presentation and scoring procedure is adopted for selection of the best potential candidate in class. The criteria include target appropriateness to lung SCC, drug/biomarker understanding, preclinical data, pharmacodynamics and pharmacokinetics, toxicity, and clinical data with proof of principle in the biomarker-selected population.
According to the results of the futility analysis in the phase II portion, the sub-studies can be quickly closed or move to a phase III registration trial. This strategy significantly reduces time, number of patients, and cost needed to bring promising agents to the clinical setting. The sub-studies are based on the same protocol design and statistical assumptions; therefore, all investigational agents are tested in a comparable manner. The addition of new sub-study populations affects the estimated biomarker overlap prevalence but does not alter the overall design or statistical assumptions.
The use of a common and detailed genotype platform facilitates broad screening and efficient allocation of patients to biomarker-specific sub-studies. The presence of a “non-match” arm, allow for all eligible patients to be accrued. This platform also offers opportunities for exploratory studies incorporating NGS results and clinical outcomes, in order to identify additional predictive biomarkers as well as potential resistance mechanisms. The tissue and blood banking from patients screened for LUNG-MAP will represent one of the largest repositories of squamous cell lung cancer. The master protocol is flexible and adaptable, allowing for incorporation of “new standards” in an evolving field.
Adaptability of the framework
It was with great excitement that the Lung-MAP group of investigators saw the approval of nivolumab (Opdivo) as second line therapy of lung SCC, in the same research space occupied by Lung MAP (17). The Lung-MAP team had recognized the potential of immunotherapies as treatments for lung cancer in its early design by choosing an investigational checkpoint inhibitor from AstraZeneca/MedImmune to be part of the inaugural launch of the trial. The flexibility of the Lung-MAP study design allowed the study team to modify the trial to allow patients to participate when they have received not just one prior, but now two or more lines of systemic therapy, thus allowing patients to receive nivolumab prior to entry on the trial. It also prompted a change in the design of the non-match arm as described below.
The study is also allowing patients to be screened while receiving first-line therapy (pre-screening), which will facilitate and expedite enrollment upon progression. Another important change is the modification of the non-match sub-study to single arm treatment with MEDI4736 an anti-PDL1 monoclonal antibody. An additional change in the trial was the closure of one of the initial sub-studies (S1400E), rilotumumab vs. erlotinib because the manufacturer, Amgen, withdrew the drug from its phase III study in gastric cancer on observation of toxicity that was not outweighed by efficacy. The revised study schema is shown in Figure 2. Clearly, as nivolumab becomes second line standard of care therapy for lung SCC, changes are being made to the current non-match sub-study and consideration will also be given to changes in the control arm for the biomarker matched targeted therapy sub-studies.
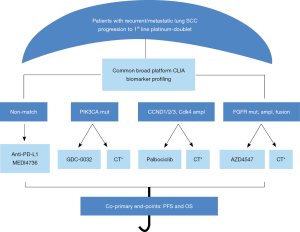
As of July 2015, a year after activation of the study, 330 patients have been enrolled in the master protocol and 261 have been assigned to a sub-study. Sub-study A (non-match arm) is leading in terms of accrual with 90 patients enrolled and an estimated complete accrual in the fall of 2015. The estimated time to complete accrual for the biomarker-matched sub-studies will range between 14 and 36 months.
Conclusions
LUNG-MAP is an “umbrella,” tumor specific protocol, inspired on BATTLE and I-SPY2, but with unique characteristics. It does not use adaptive randomization. The target therapy being tested may not have been validated for a specific biomarker, but have a high potential to be active in the molecular defined population based on promising phase I trials results and strong rationale. The turnaround time of the genetic screening is short and pre-screening is allowed, which is essential in the setting of refractory lung SCC. It also combines the learning (phase II) and confirmatory (phase III) phases into a single seamless phase II-III trial. Counting the patients from the phase II stage towards the phase III portion decreases the total sample size needed. This strategy diminishes administrative burden, cost, and time to get effective therapies to patients, as each sub-studies has the potential for drug registration. It is also appealing for investors and pharmaceutical companies as the phase II study results can be published while the phase III portion is ongoing. Indeed the major challenges to the development of the protocol were logistic in nature rather than lack of engagement and enthusiasm by academia and pharmaceutical industry and the protocol has actually been embraced by community practices that have been leading the accrual. The Master Protocol is flexible, allowing sub-studies to enter on a rolling basis as other closes. It also will create a biorepository of refractory lung SCC tissue, blood, and imaging, which will allow future translational research studies. It is frequently revised to accommodate advances made in the evolving field. Finally, it has a great potential to implement personalized cancer care in an organized, cost-effective and timely manner that will ultimately impact the lives and bring hope for innumerous metastatic lung SCC patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [PubMed]
- Dancey JE. Epidermal growth factor receptor inhibitors in non-small cell lung cancer. Drugs 2007;67:1125-38. [PubMed]
- Shaw AT, Engelman JA. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:2537-9. [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [PubMed]
- Planchard D, Kim TM, Mazières J, et al. Dabrafenib in patients with BRAF V600E-mutant advanced Non-Small Cell Lung Cancer (NSCLC): a multicenter, open-label, Phase II trial. Ann Oncol 2014;25:LBA38.
- Besse B, Doria J, Yao B, et al. Neratinib with or without temsirolimus in patients with non-small cell lung cancer (NSCLC) carrying HER2 somatic mutations: An international randomized phase II study. Ann Oncol 2014;25:LBA39.
- Gautschi O, Pall G, Schultheis A, et al. Lung adenocarcinoma with RET fusion: early experience with diagnosis and targeted therapy. J Thorac Oncol 2014.S7-S52.
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [PubMed]
- Heist RS, Mino-Kenudson M, Sequist LV, et al. FGFR1 amplification in squamous cell carcinoma of the lung. J Thorac Oncol 2012;7:1775-80. [PubMed]
- Dilts DM, Cheng SK, Crites JS, et al. Phase III clinical trial development: a process of chutes and ladders. Clin Cancer Res 2010;16:5381-9. [PubMed]
- Comis RL, Miller JD, Aldigé CR, et al. Public attitudes toward participation in cancer clinical trials. J Clin Oncol 2003;21:830-5. [PubMed]
- Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov 2011;1:44-53. [PubMed]
- Available online: http://www.ispy2trial.org/about/i-spy-2-trial
- Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [PubMed]
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401-4. [PubMed]
- Redman MW, Goldman BH, LeBlanc M, et al. Modeling the relationship between progression-free survival and overall survival: the phase II/III trial. Clin Cancer Res 2013;19:2646-56. [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [PubMed]


