The ALTERNATE trial: assessing a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2− invasive breast cancer
Introduction
Estrogen contributes to the development and progression of breast cancer through the carcinogenic effects of estrogen metabolites and stimulation of the estrogen receptor (ER) signaling pathways (1). In post-menopausal women, estrogens are largely a result of the conversion of adrenal androgens by the aromatase enzyme in adipose tissues. Estrogen signaling is dependent upon the binding of estrogen to its receptors, ERα and ERβ (2,3). Approximately 70-80% of all breast tumors express ERα protein (4). Endocrine treatment strategies for postmenopausal women with ER positive (ER+) breast cancer have been developed: to bind to the ERs without activating it but preventing estrogen from binding to these receptors [selective ER modulators (SERM)], to degrade ER (fulvestrant), or to block estrogen biosynthesis by inhibiting aromatase [aromatase inhibitors (AIs)]. These strategies been demonstrated to be efficacious in neo-adjuvant, adjuvant, and metastatic disease settings for postmenopausal women with ER+ breast cancer (3,5,6); in the adjuvant setting when combined with ovarian function suppression for premenopausal women with early stage ER+ breast cancer (6-8) and in the prevention setting for postmenopausal women at high risk of developing breast cancer (6,9,10). However, there is variability in both tumor response and treatment tolerability. There is an unmet clinical need to identify patients with endocrine sensitive disease for whom adjuvant chemotherapy could be avoided and to investigate the underlying drivers of endocrine resistant tumors to inform the development of targeted agents to prevent disease recurrence.
Neoadjuvant endocrine therapy (ET) in postmenopausal women with locally advanced ER+ breast cancer
Neoadjuvant ET in postmenopausal women with locally advanced ER+ breast cancer may result in a reduction in tumor size thereby either improving the chances of breast conserving surgery or rendering an inoperable tumor operable. Moreover, neoadjuvant ET provides the opportunity for interrogation of pre- and post-treatment tumor specimens to assess tumor responsiveness to ET in the early disease setting.
Two randomized neoadjuvant clinical trials (IMPACT and PROACT) enrolled postmenopausal women with newly diagnosed ER+ invasive breast cancer whose extent of disease was considered to require a mastectomy or to be inoperable to assess whether: (I) the clinical response rate; or (II) the rate of conversions to breast conserving surgery among women differed between types of endocrine therapies (11,12). The PROACT trial randomized patients between tamoxifen (20 mg daily) and anastrozole (1 mg daily) for 16 weeks prior to surgery and then post-surgery for a total of 5 years. Concurrent chemotherapy was allowed. The IMPACT trial randomized patients to tamoxifen (20 mg daily), anastrozole (1 mg daily) or the combination of tamoxifen and anastrozole for 12 weeks prior to surgery then post-surgery for a total of 5 years. Concurrent chemotherapy was not allowed. The findings among the patients who received only ET on the PROACT trial were similar to that of the IMPACT trial. No significant differences were seen in the clinical response rate or the percentage of women who became candidates for breast-conserving surgery between anastrozole and tamoxifen.
Suppression of the proliferation marker, Ki67 after short term exposure to NET
The design of the IMPACT trial mirrored that of the ATAC trial which randomized postmenopausal women with newly diagnosed breast cancer to 5 years of adjuvant treatment with tamoxifen (20 mg daily), anastrozole (1 mg daily) or their combination (13). One of the aims of the IMPACT study was to assess whether changes in the proliferation maker, Ki67, after 2 or 12 weeks of neoadjuvant ET would reflect differences in long term outcomes seen in the ATAC trial. The IMPACT trial found that the suppression of the proliferation marker Ki67 after 2 and 12 weeks of treatment was significantly greater with anastrozole than with tamoxifen (14). This finding mirrored that of the ATAC trial where both disease-free survival and time to recurrence were found to be significantly increased with anastrozole relative to tamoxifen. IMPACT trial also demonstrated that high Ki67 expression levels after 2 weeks of neoadjuvant ET (anastrozole, tamoxifen or their combination) was associated with poorer recurrence-free survival (15), raising the possibility that changes in tumor biomarkers after short-term exposure to ET may improve our ability to predict long term outcomes in individual patients.
Greater suppression of the proliferation marker Ki67 after 16 weeks of neoadjuvant treatment with an AI relative to a SERM was also seen in P024, a randomized neo-adjuvant randomized, double-blind clinical trial comparing letrozole (2.5 mg daily) to tamoxifen (20 mg daily) in postmenopausal women with hormone receptor positive primary invasive breast cancer who were not eligible for breast conserving surgery (16). Patients enrolled onto P024 continued to receive their assigned endocrine treatment for a total of 5 years post-surgery. Utilizing the long term outcomes of these patients, Ellis et al. found that pathologic tumor stage, pathologic nodal stage, surgical specimen Ki67 level, and ER Allred score were independently associated with both relapse-free survival (RFS) and breast cancer specific survival (17). The preoperative endocrine prognostic index (referred to as the PEPI score, Table 1) was developed based on these findings. Outcome data from the IMPACT trial was used to assess the validity of the PEPI score as a prognostic index for RFS. Patients were classified into 3 PEPI risk groups (0 vs. 1-3. vs. 4+). RFS was indeed found to differ significantly among these groups (log rank P=0.002). Moreover, in both trials, patients with a PEPI score of 0 (pT1/2, pN0, Ki67 ≤2.7%, Allred score 3-8) had an extremely low risk of relapse.
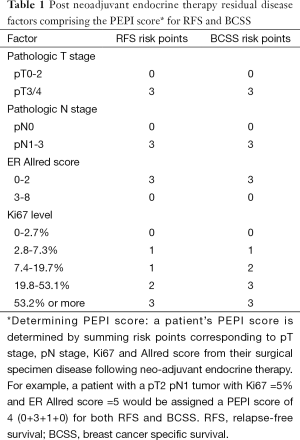
Full table
From these studies, questions arose as to whether postmenopausal women with ER positive invasive breast cancer that was either operable or potentially operable who have a PEPI score of 0 after neoadjuvant AI therapy could forgo adjuvant chemotherapy
Z1031 was a randomized neoadjuvant phase II screening trial in post-menopausal women with clinical stage II/III ER+ breast cancer designed to determine which endocrine agent (anastrozole, letrozole or exemestane) or subset of agents should be recommended for future testing against chemotherapy in the neo-adjuvant setting based on differences in clinical response rates (using WHO criteria) after 16 weeks of treatment (18). Both letrozole and anastrozole met the criteria for recommendation for further study. However, no significant differences were found among these 3 endocrine treatments in terms of Ki67 suppression after 16 weeks of treatment or PEPI-0 rate.
An extension of Z1031 (Z031B) examined whether post-menopausal women with clinical stage II/III ER+ breast cancer and a tumor Ki67 >10% after 4 weeks of anastrozole or letrozole treatment would benefit from switching to neoadjuvant chemotherapy (19). A second objective was to examine long-term outcomes in women with a tumor Ki67 ≤10% after 4 weeks of NET and a PEPI score =0 at the completion of 16 weeks of NET who do not receive adjuvant chemotherapy. Both the 4-week tumor biopsy specimen and surgical specimen were submitted to a central laboratory for a Ki67 determination and results were returned to the submitting sites within 14 days of submission to ensure timely decision making. Among the 245 women enrolled on Z1031B, 35 had a 4 week Ki67 >10% and switched to neoadjuvant chemotherapy. There were two (5.7%, 95% CI: 0.7-19.1%) pathologic complete responses among these 35 women. Long term outcome data are maturing. This study demonstrated the feasibility of conducting biomarker directed triage trials in the neoadjuvant setting.
The impact of neoadjuvant ET on the proliferation marker, Ki67, has also been studied in the estrogen-receptor antagonist, fulvestrant, which binds, blocks and accelerates the degradation of the ER. Trial 0057, a double-blind, randomized phase II neoadjuvant clinical trial in postmenopausal women with ER+ primary cT1-3 breast cancer examined the changes in ER and Ki67 expression pre and post 2 to 3 weeks of treatment with either the combination of fulvestrant (500 mg day 1) and anastrozole (1 mg/day days 14-21); fulvestrant with anastrozole placebo; or anastrozole with fulvestrant placebo (20). Ki67 expression was found to be significantly reduced from pre-treatment measurements for each of these treatment groups. Moreover, amount of reduction in Ki67 expression was not found to differ with respect to treatment. Also, the reduction in ER expression was significantly greater with fulvestrant alone or in combination with anastrozole than with anastrozole alone.
ALTERNATE trial design considerations
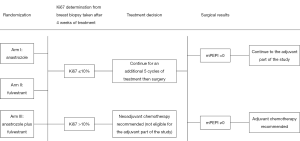
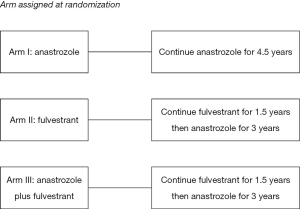
The Alliance for Clinical Trials in Oncology cooperative group designed a randomized phase III clinical trial (ALTERNATE trial) in women with cT2-4 N0-3 M0 ER+/Her2− invasive breast cancer to assess a biomarker driven treatment strategy based on Ki67 values following 4 and 12 weeks of neoadjuvant ET and the PEPI score to identify women at low risk of disease recurrence (Figures 1,2). The treatment strategies under investigation in this trial are: (I) anastrozole administered by mouth 1 mg days 1-28 for 6 28 day-cycles, surgery, and then anastrozole by mouth 1 mg daily for 4.5 years; (II) fulvestrant 500 mg administered intramuscularly days 1 and 15 of the first 28 day cycle and then day 1 of the 5 remaining 28 day neoadjuvant ET cycles; surgery; fulvestrant day 1 of first 18 months post-surgery followed by anastrozole by mouth 1 mg daily for 3 years; and (III) the combination of anastrozole and fulvestrant employing the same administrative schedule as in the single agent arms for the neoadjuvant portion; surgery and then fulvestrant day 1 of first 18 months post-surgery and anastrozole by mouth 1 mg daily for 3 years. It is recommended that women with tumor Ki67 >10% on breast biopsy after 4 weeks (mandatory) or 12 weeks (optional) of neoadjuvant ET switch to neo-adjuvant chemotherapy. Also, women having completed 6 months of neoadjuvant ET and found to have pT3/4 or pN1-3 or Ki67 >2.7% residual disease at surgery are recommended to receive adjuvant chemotherapy of their physician’s choosing.
Within this overarching goal, a number of questions concerning endocrine resistance in both the neoadjuvant and adjuvant settings will be addressed. Endocrine resistance in the neoadjuvant disease setting is defined as one of the following events: (I) Ki67 >10% after 4 weeks of neoadjuvant ET; (II) Ki67 >10% after 12 weeks of neoadjuvant ET; (III) radiographic confirmation of progressive disease during neoadjuvant ET; (IV) surgical findings of pT3/4 or pN1-3 or Ki67 >2.7% residual disease; or (V) discontinuation of neoadjuvant ET for any reason. The primary objective for the neoadjuvant portion of the ALTERNATE trial is to determine whether endocrine resistant rate (ERR) with fulvestrant alone or with fulvestrant plus anastrozole is less than that for anastrozole alone. Secondary aims include examining differences in surgical outcomes, clinical and radiographic response rates and safety profile between the three treatment arms. Correlative objectives include examining the degree of tumor Ki67 suppression in each treatment arm as well as evaluating tumor tissue, serum, and plasma specimens collected prior to neoadjuvant ET, after 4 weeks of neoadjuvant ET, and at surgery to gain insights into signaling pathways associated with endocrine resistance.
A comparison of the endocrine sensitivity rate (1-ERR or ESR) among the first 440 patients randomized to each treatment arm will be used to determine whether any of the fulvestrant containing arms should be closed to further enrollment. Consideration for retaining a fulvestrant containing treatment arm will be based on whether its ESR is at least 10% greater than that for anastrozole alone. The sample size for these 2 pairwise comparisons was determined assuming the ESR for the anastrozole arm would be similar to that the anastrozole arm of Z1031B, namely, 34%. For a given fulvestrant containing arm, a one-sided alpha =0.025 chi-square test of the difference in two independent binomial proportions will have a 82% change of detecting a 10% or greater increase in ESR with this fulvestrant containing regimen, when the ESR with anastrozole alone is at most 34%. In addition, 3 interim analyses are planned to assess futility based on the conditional probability (under the alternative hypothesis) of declaring a fulvestrant containing regimen having at least a 10% higher endocrine sensitivity rate than anastrozole at the final analysis (440 pts per regimen) given the endocrine sensitivity findings to that interim analysis time point.
The adjuvant portion of the ALTERNATE trial addresses questions concerning the clinical outcomes of patients considered to be at low risk of disease recurrence after neoadjuvant ET who do not receive adjuvant chemotherapy but continue to receive ET for an additional 4.5 years. Determination of whether a patient is at low risk of disease recurrence is based on 4-week Ki67 level and a modification of the PEPI score. Since fulvestrant down-regulates ER expression and a tumor rendered ER− by fulvestrant may not reflect a poor prognosis, ER Allred score results are not be included in the modified PEPI score (mPEPI). Women are classified as being at a low risk for disease recurrence if their Ki67 ≤10% after 4 weeks of neoadjuvant ET and mPEPI =0 (that is, pT0-2/pN0/Ki67 ≤2.7% residual disease). The primary endpoint of the adjuvant portion of this trial is recurrence-free survival (RFS) defined as the time from surgery to the first of the following disease events: invasive ipsilateral breast tumor recurrence, local/regional recurrence, distant recurrence or death due to any cause.
The enrollment period for this trial will depend upon whether none, one or both of the fulvestrant containing arms are found to have a favorable ESR relative to that of anastrozole as well as the finding from the Z1031B trial that 34% of the women receiving neoadjuvant ET had both a week 4 Ki67 ≤10% and mPEPI =0. The benchmark for considering this this biomarker driven strategy to be effective for a given treatment arm relies on the results of Southwest Oncology Group 8814 trial which reported that the 5-year disease-free survival rate with tamoxifen was 91% among women with pT1-3, N1 ER+/Her2− breast cancer and an Oncotype DX recurrence score ≤25 (21). As such, this biomarker driven strategy will be considered effective for a given treatment arm if its 5-year recurrence-free survival is not less than 90%. If only the anastrozole arm is to be assessed and 940 are women are randomized to anastrozole over a 5-year period (yielding 320 women with both a week 4 Ki67 ≤10% and mPEPI =0) and followed a minimum of 4 years after the close of enrollment, a one sample α =0.025 non-parameter Brookmeyer-Crowley type one sample test will have a 90% chance of rejecting that the 5-year RFS rate is 95% or more when the true 5-year RFS rate is at most 90% (22,23). If one or more of the fulvestrant containing arms are to be assessed as well, the enrollment period and/or the follow-up will be increased to ensure sufficient power to assess this primary endpoint. No interim analyses are planned for the adjuvant phase of this trial.
Trial status
This trial opened to enrollment on December 13, 2013, shortly after the merger of the American College of Surgeon Oncology Group (ACSOG), Cancer and Leukemia Group B (CALGB) and the North Central Cancer Treatment Group (NCCTG) into the Alliance for Clinical Trials in Oncology. A number of changes accompanied this merger which increased the need to provide participating sites additional materials to increase awareness of newly instituted systems and procedures impacting the conduct of this trial. An educational slide set was prepared to provide the rationale behind the clinical and correlative objectives. An instructional video was produced to illustrate tissue and blood sample procurement and processing procedures; discuss the biopsy/shipping kit contents and review shipping instructions. Both a Physician Fact Sheet and a Patient Brochure were developed. A monthly newsletter is sent to all participating sites describing any protocol changes, addressing frequently asked questions, and providing updated enrollment numbers. The Study Chair, Community Oncology Co-chair, statistical team, and data manager also hold a teleconference monthly with the Clinical Research Associates of participating sites to answer questions and gather information concerning issues they are encountering as they navigate the protocol procedures and the new Medidata RAVE data submission application.
The accrual rate was been climbing with 236 women have enrolled as of August 10, 2015.
Conclusions
The ALTERNATE trial provides the unique opportunity to prospectively validate a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2− invasive breast cancer and to examine the signaling pathways that lead to endocrine resistance.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bhatnagar AS. The discovery and mechanism of action of letrozole. Breast Cancer Res Treat 2007;105 Suppl 1:7-17. [PubMed]
- Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 2007;87:905-31. [PubMed]
- Chumsri S, Howes T, Bao T, et al. Aromatase, aromatase inhibitors, and breast cancer. J Steroid Biochem Mol Biol 2011;125:13-22. [PubMed]
- Keen JC, Davidson NE. The biology of breast carcinoma. Cancer 2003;97:825-33. [PubMed]
- Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. Int J Clin Pract 2007;61:2051-63. [PubMed]
- Ingle JN, Dowsett M, editors. Advances in Endocrine Therapy of Breast Cancer. New York: Marcel Dekker, 2004.
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011;364:2381-91. [PubMed]
- Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 2014;383:1041-8. [PubMed]
- Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014;371:107-18. [PubMed]
- Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 2015;372:436-46. [PubMed]
- Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative "Arimidex" Compared to Tamoxifen (PROACT) trial. Cancer 2006;106:2095-103. [PubMed]
- Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108-16. [PubMed]
- Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 2005;365:60-2. [PubMed]
- Dowsett M, Smith IE, Ebbs SR, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res 2005;11:951s-8s. [PubMed]
- Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 2007;99:167-70. [PubMed]
- Ellis MJ, Ma C. Letrozole in the neoadjuvant setting: the P024 trial. Breast Cancer Res Treat 2007;105 Suppl 1:33-43. [PubMed]
- Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380-8. [PubMed]
- Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J Clin Oncol 2011;29:2342-9. [PubMed]
- Ellis MJ, Suman V, McCall L, et al. Abstract PD07-01: Z1031B Neoadjuvant Aromatase Inhibitor Trial: A Phase 2 study of Triage to Chemotherapy Based on 2 to 4 week Ki67 level > 10%. Cancer Res 2012;72:PD07-01.
- Robertson JF, Dixon JM, Sibbering DM, et al. A randomized trial to assess the biological activity of short-term (pre-surgical) fulvestrant 500 mg plus anastrozole versus fulvestrant 500 mg alone or anastrozole alone on primary breast cancer. Breast Cancer Res 2013;15:R18. [PubMed]
- Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55-65. [PubMed]
- Brookmeyer R, Crowley JJ. A confidence interval for the median survival time. Biometrics 1982;38:29-41.
- One Sample-Nonparametric Survival. Available online: http://www.swogstat.org/stat/public/one_nonparametric_survival.htm