Prognostic scores for brain metastasis patients: use in clinical practice and trial design
Introduction
Brain metastasis (BM) is the most common type of intracranial malignancies. Lung, breast cancer and melanoma are the primary malignancies that contribute up to 80% of BM (1). Given the high incidence of asymptomatic BM it is hard to estimate the true prevalence but various studies estimate that approximately 10-25% of patients with cancer eventually develop BM (2-4). Until recently the median overall survival for patients with BM has been dismal and most patients survive 6 months after diagnoses (5). With the advent of sensitive imaging modalities and multiple treatment options, the prognosis of at least a select group of patients with BM has improved significantly. BM is a heterogeneous group with varied response to treatment and survival. Therefore it is important to consider the various factors affecting the prognosis of patients with BM prior to therapeutic decision making.
Prognostic factor is defined by National Cancer Institute (NCI) as a situation or condition, or a characteristic of a patient, that can be used to estimate the chance of recovery from a disease or chance of the disease recurring (6). Prognostic indices have been utilized in different malignancies with the aim to improve the understanding of patients’ prognosis and aid the clinical and therapeutic decision making (7). Furthermore, prognostic scores play a crucial role in patient selection, stratification and randomization in clinical trials. Multiple studies, albeit retrospective in nature, have elucidated prognostic factors and recommended prognostic scoring systems for BM (Table 1).
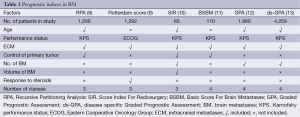
Full table
Recursive Partitioning Analysis (RPA) classes
Gaspar et al. in 1997 evaluated 1,200 patients from three RTOG trials (79-16, 85-28, and 89-05) who were treated with whole brain radiation therapy (WBRT) for BM (8). Overall, Karnofsky performance status (KPS), age, control of primary and the status of extracranial disease were found to impact survival. Using RPA, three classes were formulated; patients with KPS ≥70, age less than 65 years, controlled primary and no other systemic metastasis had the best survival (median, 7.1 months) and were grouped in class I. Class III on the other hand was associated with worst prognosis (median, 2.3 months) and included all the patients with KPS less than 70. The rest of the patients were grouped into class II and had intermediate survival (median, 4.2 months). The number of BM was a significant factor for survival in the univariate analysis but was found to be statistically insignificant in the final RPA analysis. Several other retrospective studies have validated the RPA classification (14-16). RPA classification was further verified in patients treated with stereotactic radiation (SRS) or surgery (17,18). Additionally, RPA was evaluated and validated in breast cancer, non-small cell, small cell lung cancer, and melanoma patients (19-25). However this analysis although a step in the right direction had some limitations; the eligibility criteria for these three trials was different. For example, KPS was ≥40 in RTOG 79-16 trial compared to KPS >60 in RTOG 85-28 and 89-05. The patients were also treated with different doses and schedules of WBRT. Additional inherent deficiency of RPA index is that it is best for patients treated with WBRT showing consistent survival within the same class, across different studies but same may not be true for patients treated with other modalities like surgery and SRS. Another major limitation was definition of class III. Class III contained all patients with KPS <70, which might result from different etiologies, including BM, systemic disease, other medical conditions. In an attempt to better define RPA class III, Lutterbach et al. divided it into three separate classes; class IIIa included age <65 years, controlled primary, and single BM, whereas class IIIc included age >65 years, uncontrolled primary, and multiple BM (26). Class IIIb had all other patients in the class, however the modification by Lutterbach and colleagues has not been widely accepted (7). Class II also represents a heterogeneous group and there have been attempts to further subdivide class II patients. Yamamoto et al. studied RPA in a large Japanese retrospective review of 3,753 patients and subdivided RPA class II (27). Four factors predicted survival among the 1,414 patients in RPA class II; KPS (90 to 100 vs. 70 to 80), number of BM (solitary vs. multiple), primary tumor status (controlled vs. uncontrolled) and extracranial metastases (ECM) (absent vs. present). Even though RPA has been widely accepted and used in multiple clinical trials in the past, multiple indices have been proposed to address the above-mentioned limitations.
Rotterdam score
In 1999, with the aim to improve the existing prognostic score, a single institution database of 1,292 patients was analyzed (9). In addition to the established prognostic factors (KPS, age, control of primary and the status of extracranial disease), response to steroids, serum lactate dehydrogenase, sex in lung primary and site of primary tumor were found to be significant in this analysis. However, the final scoring system included KPS, response to steroids and extracranial disease. Most centers do not have data available for response to steroids making this scoring system difficult to use in clinical practice.
Score Index For Radiosurgery (SIR)
To better elucidate the prognostic factors among BM patients treated with SRS, “SIR” which is composed of six variables; age, KPS, extra-cranial disease status, number of BM, volume of the largest BM, location of BM and post radiosurgery WBRT was proposed (10). The SIR was more reliable than RPA in predicting survival among 65 patients treated with radiosurgery in this analysis. Several groups validated the SIR in patients subjected to surgery, WBRT with or without SRS (11,28-30). The detailed work up needed to assess the systemic disease limited the wide spread use of this prognostic index (7).
Basic Score for Brain Metastases (BSBM)
Lorenzoni et al. proposed a new scoring system that compared RPA with SIR called BSBM (11). With the aim to keep the scoring simple, the BSBM included three factors; KPS, control of primary tumor and presence of extracranial disease. In the analysis of 110 BM patients treated with SRS, BSBM and SIR were both accurate in prognostication. The BSBM was further evaluated in patients receiving WBRT with surgery and WBRT with or without SRS (5,31). BSBM has been advocated as a convenient, easy to use prognostic index that has same definition of extracranial disease as the RPA.
Graded Prognostic Assessment (GPA)
RTOG 9508, a randomized trial of WBRT with or without SRS boost for patients with one to three BM concluded that number of BM was significant for prognosis (32). However RPA, BSBM, and Rotterdam score did not include number of BM in the prognostic score. In 2007, a new scoring system called the GPA was proposed (12). The GPA incorporated four factors: age, KPS, ECM and number of BM that impact prognosis in BM. Each factor was given a score of 0, 0.5 or 1.0 and GPA was calculated a sum score of all four factors. For example, a 58-year-old patient with a KPS of 70, 4 BM and presence of ECM will have a GPA of 1 (age-0.5, KPS-0.5, BM-0 and ECM-0). This GPA was compared with RPA, BSBM and SIR in a retrospective study of 1,960 patients from five RTOG trials (RTOG 7916, 8528, 8905, 9104, and 9508). The GPA had four groups, the GPA 0-1 with median survival of 2.6 months; GPA 1.5-2.5 with survival of 3.8 months; GPA 3 with median survival of 6.9 months and GPA 3.5-4.0 with the best median survival of 11 months. All the indices compared were prognostic with GPA being as prognostic as RPA. Since then various studies have validated the GPA (33-35). The authors concluded that “GPA is least subjective, most quantitative and easiest to use of the four indices (RPA, SIR, BSBM, and GPA)”. Since that time GPA has become one of the most commonly used prognostic index in clinical practice.
Disease specific Graded Prognostic Assessment (ds-GPA)
It has been long known that BM from different primaries responds differently to radiation. The primary tumor type was not considered in any of the previous prognostic indices. Golden et al. analyzed different prognostic indices among 479 newly diagnosed BM patients and concluded that the prognosis varied with the primary tumor site (36). With the aim to identify disease specific prognostic factors Sperduto et al. evaluated 4,259 patients from 11 different institutions (13). Age, KPS, number of BM and sites of ECM strongly predicted survival in lung (non-small cell and small cell) cancer. Age, KPS, subtype were the prognostic factors that impacted survival in breast cancer. Only age and KPS were significant factors predicting survival in melanoma and renal cell cancer patients. Among GI cancer patients only KPS predicted survival.
A number of investigators have proposed different prognostic indices to better define the prognosis of breast cancer patients. Sperduto and colleagues reported their work on 400 breast cancer patients with BM (37). Genetic subtypes of breast cancer had significant effect in prognosis of patients with BM. The basal subtype [ER/PR negative and human epidermal growth factor receptor 2 (HER2) HER2 negative] patients had the shortest survival whereas the luminal B subtype (ER/PR positive and HER2 positive) patients had the best survival. The genetic subtypes, age, and KPS were included in the revised ds-GPA for breast cancer. Weightage based system was used to assign points for each prognostic factor. Basal, luminal A, HER positive and luminal B subtypes were assigned 0, 1.0, 1.5, and 2.0 points respectively. Age as a prognostic factor had the lowest weightage therefore patients older than 60 years were assigned 0 points and younger than 60 years were assigned 0.5 points. KPS of ≤50, 60, 70-80, 90-100 were given 0, 0.5, 1.0 and 1.5 points respectively. The ds-GPA had four groups with total scores of 0.5-1.0, 1.5-2.0, 2.5-3.0, and 3.5-4.0 with median survival of 3.4, 7.7, 15.1, and 25.3 months respectively. Other breast cancer specific prognostic indices proposed include work from Nieder (38), Le Scodan (21), Park (39) and Claude (40) (Table 2).
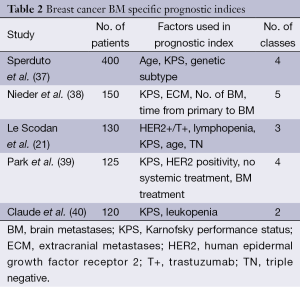
Full table
Discussion
The management of metastatic cancer is changing rapidly with different newer agents targeting the various driver mutations and immunotherapeutic agents leading to prolonged survival. With the improved survival and newer sensitive diagnostic modalities the incidence of BM is on the rise. Traditionally BM patients are treated with WBRT, SRS, surgery or a combination of them. Systemic therapy had limited role in the treatment of BM in the past, but with advent of agents with better CNS activity they will be important part of management of these patients. Lapatinib has clinical activity in HER2 positive breast cancer BM patients (41), alectinib has shown promise in treatment of BM in anaplastic lymphoma kinase (ALK) rearranged non-small cell lung cancer (42). In melanoma patients with BRAF mutation and BM, a number of BRAF inhibitors such as vemurafenib and dabrafenib and immunotherapeutic agents have shown intracranial activity (43). With all the available therapeutic options it is important to be aware of the prognosis of patients with BM in order to make the best treatment decision for them. A recent study by Kondziolka et al. attempted to address the accuracy in predicting survival in patients with BM using prognostic scores (44). Clinical, radiologic, and primary tumor data on 150 patients with BM was provided to a group of expert neurosurgeons, radiation oncologists, and medical/neuro-oncologists and they were asked to predict survival. The predicted survival data generated by the experts was compared with the actual survival. A total of 2,700 predictions were made, of which 1,226 (45%) were off by 6 months and 18% were off by more than one year. Radiation oncologists and neurosurgeons overestimated the survival while medical/neuro oncologists under estimated the survival. Overall a considerable variation in survival prediction was noted in the study supporting a need for a better prognostic tool or index. The prognostic indices also play an important role in balancing the cost of treatment and providing realist expectations to the patients’ and the caregivers (45). The patients with poor prognosis can be offered supportive care and those with good prognosis can be treated with aggressive strategies that often employ multimodality treatment. The prognostic scores play a vital role in designing clinical trials as well. The patients with similar prognosis should be stratified together in trials, to limit confounding factors and improve the applicability and validity of trials.
Most the prognostic scores have some inherent limitations, for e.g., RPA and BSBM do not include number of BM an important prognostic factor. The calculation of SIR score requires treatment factors like tumor volume at radiosurgery restricting its use in patients subjected to SRS only. The major limitation of GPA was the lack of consideration of differences in primary tumor characteristics. The ds-GPA was formulated for BM from different primary malignancies but did not consider the role of mutations. Age and KPS been proven to have prognostic significance in multiple studies across various tumor subtypes. Number of BM has been prognostic in multiple tumor subtypes.
In recent year more studies have attempted to clarify the role of mutations or tumor subtypes similar to the breast specific GPA performed by Sperduto and colleagues. Johung et al. evaluated the role of driver mutation genotype in predicting recurrence among NSCLC BM patients treated with radiosurgery (46). Four hundred and ninety-six BM were followed; none of the patients with EGFR mutation and EML4-ALK translocation had in-field recurrence, whereas 18% of patients with KRAS mutation and 19% without these mutations had in-field recurrence. Even though survival analysis was not reported, this study provided valuable insight into the impact of mutations on radiation efficacy for e.g., EGFR and EML4-ALK mutant tumors are more radiosensitive as compared to BM that harbor KRAS mutation. Further investigation is needed to define the prognostic impact of various mutations such as BRAF in melanoma, EGFR and ALK in non-small cell lung cancer and KRAS mutation in both non-small cell lung cancer and gastrointestinal malignancies. Prognostic value of various other tumor related characteristics has been evaluated in a number of retrospective studies. Spanberger et al. assessed Ki-67 index, hypoxia induced factor 1 alpha (HIF1a) expression, peritumoral edema and microvascularization patterns in 219 patients who underwent resection of BM and tumor tissue was available for evaluation (47). In addition to GPA, peritumoral edema was the only factor associated with prognosis. The role of imaging characteristics to predict survival were studied in 65 patients with single BM (48). The data on preoperative MRI features such as diffusion weighted imaging (DWI) signal intensities and apparent diffusion coefficient (ADC) maps were correlated with survival. Preoperative DWI had a significant impact on overall survival in this study. Incorporation of these radiological parameters (e.g., edema, DWI) may help to increase accuracy of prognostic scores. However, these radiological factors need further evaluation in large data sets of BM to validate their role in predicting prognosis in BM.
Another limitation of prognostic indices is that all the factors are derived based on survival and there is no scores that address endpoints other than survival. In recent times a number of trials have used time to neurologic progression or decline as primary endpoint. One avenue for future directions would be development of prognostic models that can provide estimates of time to neurologic progression or decline and also focus not only on survival but is able to differentiate between death resulting from systemic cancer progression or neurological decline from BM.
Novel statistical models based nomograms have been proposed as an alternate for prognostic indices (49-51). Smart phone applications and spreadsheet calculators will increasingly be employed as the prognostic scores become more specific and complex. Prospective randomized studies are needed to develop a robust prognostic scoring system which in-turn will help in patient care and management.
Acknowledgements
Funding: MS Ahluwalia reports receiving research grants, through his institution, from Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly/ImClone Systems, Novartis, Spectrum Pharmaceuticals, and TRACON Pharmaceuticals. The work was supported by Dean and Diane Miller Family Endowed Chair in Neuro-Oncology.
Disclosure: MS Ahluwalia has served as consultant on advisory board for Caris Life Sciences, Genentech/Roche, and Incyte.
References
- Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep 2012;14:48-54. [PubMed]
- Chason JL, Walker FB, Landers JW. Metastatic carcinoma in the central nervous system and dorsal root ganglia. A prospective autopsy study. Cancer 1963;16:781-7. [PubMed]
- Fox BD, Cheung VJ, Patel AJ, et al. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am 2011;22:1-6. [PubMed]
- Brastianos HC, Cahill DP, Brastianos PK. Systemic therapy of brain metastases. Curr Neurol Neurosci Rep 2015;15:518. [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [PubMed]
- National Cancer Institute. NCI Dictionary of Cancer Terms. Available online: http://www.cancer.gov/dictionary?cdrid=44246
- Nieder C, Mehta MP. Prognostic indices for brain metastases--usefulness and challenges. Radiat Oncol 2009;4:10. [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [PubMed]
- Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 1999;43:795-803. [PubMed]
- Weltman E, Salvajoli JV, Brandt RA, et al. Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys 2000;46:1155-61. [PubMed]
- Lorenzoni J, Devriendt D, Massager N, et al. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys 2004;60:218-24. [PubMed]
- Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510-4. [PubMed]
- Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010;77:655-61. [PubMed]
- Nieder C, Nestle U, Motaref B, et al. Prognostic factors in brain metastases: should patients be selected for aggressive treatment according to recursive partitioning analysis (RPA) classes? Int J Radiat Oncol Biol Phys 2000;46:297-302. [PubMed]
- Fleckenstein K, Hof H, Lohr F, et al. Prognostic factors for brain metastases after whole brain radiotherapy. Data from a single institution. Strahlenther Onkol 2004;180:268-73. [PubMed]
- Saito EY, Viani GA, Ferrigno R, et al. Whole brain radiation therapy in management of brain metastasis: results and prognostic factors. Radiat Oncol 2006;1:20. [PubMed]
- Chidel MA, Suh JH, Reddy CA, et al. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys 2000;47:993-9. [PubMed]
- Agboola O, Benoit B, Cross P, et al. Prognostic factors derived from recursive partition analysis (RPA) of Radiation Therapy Oncology Group (RTOG) brain metastases trials applied to surgically resected and irradiated brain metastatic cases. Int J Radiat Oncol Biol Phys 1998;42:155-9. [PubMed]
- Mahmoud-Ahmed AS, Suh JH, Lee SY, et al. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys 2002;54:810-7. [PubMed]
- Viani GA, Castilho MS, Salvajoli JV, et al. Whole brain radiotherapy for brain metastases from breast cancer: estimation of survival using two stratification systems. BMC Cancer 2007;7:53. [PubMed]
- Le Scodan R, Massard C, Mouret-Fourme E, et al. Brain metastases from breast carcinoma: validation of the radiation therapy oncology group recursive partitioning analysis classification and proposition of a new prognostic score. Int J Radiat Oncol Biol Phys 2007;69:839-45. [PubMed]
- Videtic GM, Adelstein DJ, Mekhail TM, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for small-cell lung cancer-only brain metastases. Int J Radiat Oncol Biol Phys 2007;67:240-3. [PubMed]
- Gülbaş H, Erkal HS, Serin M. The use of recursive partitioning analysis grouping in patients with brain metastases from non-small-cell lung cancer. Jpn J Clin Oncol 2006;36:193-6. [PubMed]
- Rades D, Schild SE, Lohynska R, et al. Two radiation regimens and prognostic factors for brain metastases in nonsmall cell lung cancer patients. Cancer 2007;110:1077-82. [PubMed]
- Buchsbaum JC, Suh JH, Lee SY, et al. Survival by radiation therapy oncology group recursive partitioning analysis class and treatment modality in patients with brain metastases from malignant melanoma: a retrospective study. Cancer 2002;94:2265-72. [PubMed]
- Lutterbach J, Bartelt S, Stancu E, et al. Patients with brain metastases: hope for recursive partitioning analysis (RPA) class 3. Radiother Oncol 2002;63:339-45. [PubMed]
- Yamamoto M, Sato Y, Serizawa T, et al. Subclassification of recursive partitioning analysis Class II patients with brain metastases treated radiosurgically. Int J Radiat Oncol Biol Phys 2012;83:1399-405. [PubMed]
- Selek U, Chang EL, Hassenbusch SJ 3rd, et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys 2004;59:1097-106. [PubMed]
- Rades D, Pluemer A, Veninga T, et al. A boost in addition to whole-brain radiotherapy improves patient outcome after resection of 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer 2007;110:1551-9. [PubMed]
- Goyal S, Prasad D, Harrell F Jr, et al. Gamma knife surgery for the treatment of intracranial metastases from breast cancer. J Neurosurg 2005;103:218-23. [PubMed]
- Nieder C, Geinitz H, Molls M. Validation of the graded prognostic assessment index for surgically treated patients with brain metastases. Anticancer Res 2008;28:3015-7. [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [PubMed]
- Antoni D, Noël G. Radiotherapy of brain metastases according to the GPA score (Graded Prognostic Assessment). Cancer Radiother 2013;17:424-7. [PubMed]
- Tabouret E, Metellus P, Gonçalves A, et al. Assessment of prognostic scores in brain metastases from breast cancer. Neuro Oncol 2014;16:421-8. [PubMed]
- Luo J, Zhu H, Tang Y, et al. Analysis of prognostic factors and comparison of prognostic index scores in patients with brain metastases after whole-brain radiotherapy. Int J Clin Exp Med 2014;7:5217-25. [PubMed]
- Golden DW, Lamborn KR, McDermott MW, et al. Prognostic factors and grading systems for overall survival in patients treated with radiosurgery for brain metastases: variation by primary site. J Neurosurg 2008;109 Suppl:77-86. [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys 2012;82:2111-7. [PubMed]
- Nieder C, Marienhagen K, Astner ST, et al. Prognostic scores in brain metastases from breast cancer. BMC Cancer 2009;9:105. [PubMed]
- Park BB, Uhm JE, Cho EY, et al. Prognostic factor analysis in patients with brain metastases from breast cancer: how can we improve the treatment outcomes? Cancer Chemother Pharmacol 2009;63:627-33. [PubMed]
- Claude L, Perol D, Ray-Coquard I, et al. Lymphopenia: a new independent prognostic factor for survival in patients treated with whole brain radiotherapy for brain metastases from breast carcinoma. Radiother Oncol 2005;76:334-9. [PubMed]
- Kaplan MA, Isikdogan A, Koca D, et al. Clinical outcomes in patients who received lapatinib plus capecitabine combination therapy for HER2-positive breast cancer with brain metastasis and a comparison of survival with those who received trastuzumab-based therapy: a study by the Anatolian Society of Medical Oncology. Breast Cancer 2014;21:677-83. [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [PubMed]
- Long GV, Margolin KA. Multidisciplinary approach to brain metastasis from melanoma: the emerging role of systemic therapies. Am Soc Clin Oncol Educ Book 2013;393-8. [PubMed]
- Kondziolka D, Parry PV, Lunsford LD, et al. The accuracy of predicting survival in individual patients with cancer. J Neurosurg 2014;120:24-30. [PubMed]
- Sperduto PW. What is your patient's GPA and why does it matter? Managing brain metastases and the cost of hope. Int J Radiat Oncol Biol Phys 2010;77:643-4. [PubMed]
- Johung KL, Yao X, Li F, et al. A clinical model for identifying radiosensitive tumor genotypes in non-small cell lung cancer. Clin Cancer Res 2013;19:5523-32. [PubMed]
- Spanberger T, Berghoff AS, Dinhof C, et al. Extent of peritumoral brain edema correlates with prognosis, tumoral growth pattern, HIF1a expression and angiogenic activity in patients with single brain metastases. Clin Exp Metastasis 2013;30:357-68. [PubMed]
- Berghoff AS, Spanberger T, Ilhan-Mutlu A, et al. Preoperative diffusion-weighted imaging of single brain metastases correlates with patient survival times. PLoS One 2013;8:e55464. [PubMed]
- Marko NF, Xu Z, Gao T, et al. Predicting survival in women with breast cancer and brain metastasis: a nomogram outperforms current survival prediction models. Cancer 2012;118:3749-57. [PubMed]
- Graesslin O, Abdulkarim BS, Coutant C, et al. Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol 2010;28:2032-7. [PubMed]
- Barnholtz-Sloan JS, Yu C, Sloan AE, et al. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol 2012;14:910-8. [PubMed]


