New approaches in primary central nervous system lymphoma
Introduction
While primary central nervous system lymphoma (PCNSL) represents approximately 3% of all brain tumors and 2-3% of all cases of non-Hodgkin’s lymphomas (NHL), the overall incidence of this neoplasm may be increasing, particularly among persons age sixty-five years and older (1). The unique pathobiology of PCNSL is that of dissemination of aggressive NHL within the brain, cranial nerves, leptomeninges, cerebrospinal fluid (CSF), intraocular structures and spinal cord, without overt systemic disease (2,3).
PCNSL has long been associated with an extremely poor prognosis (4). In the absence of prospective data, during the 1960’s, hematologists and oncologists typically employed brain irradiation [whole brain irradiation (WBRT)] as the first intervention as a means to elicit immediate responses in patients faced with a rapidly deteriorating course. WBRT alone typically resulted in median survival for PCNSL patients of only 12 months. The next advance in the treatment of PCNSL was the recognition, in the 1970’s, of the efficacy of high-dose methotrexate (HD-MTX), initially in the treatment of recurrent disease (5,6).
A number of recent phase I/II clinical trials have documented continued and dramatic improved outcomes in PCNSL. Our goal in this review therefore is to highlight key advances in our understanding of disease biology, diagnosis, staging and therapeutic management (7-12).
Etiology
Established risk factors for CNS lymphomas, both primary and secondary, include acquired and/or congenital immunodeficiency states. PCNSL is an AIDS-defining illness associated with low CD4 cell count (<50 cells/L) and Epstein Barr virus (EBV). In systemic AIDS-related lymphomas, EBV infection of the tumor may be predictive of increased risk for secondary CNS involvement (13). Congenital immunodeficiency states such as severe-combined or common-variable immunodeficiency, ataxia-telangiectasia or Wiskott-Aldrich syndrome are associated with a ~4% risk of developing PCNSL. Post-transplant lymphoproliferative disorder (PTLD) involving the CNS develops in 1-2% of renal transplant recipients and 2-7% recipients of cardiac, lung and liver transplants. CNS PTLD is strongly associated with EBV in the setting of iatrogenic T-cell immunodeficiency induced by agents such as mycophenolate mofetil (Cell Cept) (14). Among PCNSL patients without evidence of immune suppression, EBV infection of the lymphoma is rare (15).
Pathogenesis
PCNSL is a highly infiltrative neoplasm that has been characterized as a “whole brain disease” (16). In general, the radiographic appearance of the tumor markedly underestimates disease extent and burden, and like malignant gliomas, PCNSL is not amenable to curative resection (16). One of the distinguishing histologic features of PCNSL is that of angiocentricity or angiotropism; the accumulation of lymphoma cells around small and medium-sized blood vessels, a property that likely contributes to the disruption of the blood-brain barrier and enables visualization of lesions via pathologic contrast enhancement. PCNSL usually presents as a solitary mass, typically with vasogenic edema and mass effect. The frequency of multiple lesions is increased among immune suppressed patients (17).
Intraocular disease is a common manifestation of PCNSL: ~twenty percent of PCNSL patients present with involvement of the retina, vitreous, and uveal tract. An important principle is that what is initially appreciated to be localized intraocular lymphoma (IOL) will ultimately disseminate within the brain in greater than 80% of cases, and therefore, detection of IOL mandates staging of the neuroaxis including CSF evaluation and brain MRI as well as therapies that address this risk for brain involvement (18).
Approximately 95% of PCNSL are large B-cell lymphoma; other histologies that present as PCNSL include T-cell (2%) (19), lymphoblastic, Burkitt, and marginal zone lymphomas. PCNSL is distinguished from dural-based marginal zone lymphomas as these have a distinct pathogenesis, typically do not disseminate within the brain parenchyma, and share overlapping radiographic features on MRI with meningioma (20).
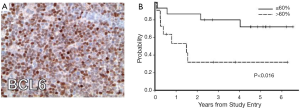
A number of investigations have recently focused on elucidation of the features of PCNSL, large cell type. Between 50% to 80% of PCNSL tumors express BCL6 by immunohistochemistry (21) and at least 95% stain positive for MUM-1; therefore the majority of PCNSL cases are of an activated B-cell immunophenotype of large-cell lymphoma (15). Immunohistochemical analysis of tumors evaluated in CALGB 50202 provided evidence that high BCL6 expression by PCNSL tumors may correlate with refractory disease and shorter progression-free and overall survival (12), thus representing a potentially useful molecular prognostic biomarker (Figure 1). Between 56-93% of PCNSL express BCL2 (15,21). Transcriptional profile studies of PCNSL identified several potential mediators of disease pathogenesis, including high MYC expression (22). Increased MYC in PCNSL was later confirmed in the recent CALGB 50202 study (12). High expression of miRNA’s involved in the MYC pathway (23), as well as MYC translocations (24) have also been demonstrated in PCNSL. Given the reproducible evidence for distinct transcriptional features in PCNSL (22,25-27) as well as the fact that the disease requires treatment regimens that are distinct from its systemic counterpart, PCNSL is recognized as a distinct subtype of large B-cell lymphoma by the WHO (28).
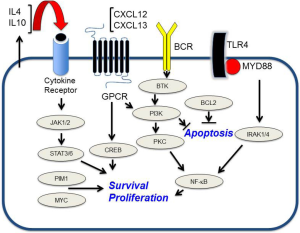
Common genomic aberrations in PCNSL include losses on chromosome 6p21 that harbor loci for HLA (24,29,30) as well as broad deletions involving chromosome 6q. Candidate tumor suppressor genes linked to deleted loci on chromosome 6q include PRDM1, a tumor suppressor and regulator of B-cell differentiation (31), PTPRK, a protein tyrosine phosphatase that participates in cell adhesion signaling events (32), and A20 (TNFAIP3), a regulator of NFκB signaling (33). Further evidence for the aberrant activation of NFκB pathways is supported by gain in DNA copy number for MALT1 (34) as well as activating mutations of CARD11 (35) and MyD88. The activating exchange of leucine to proline at position 265 of MyD88 may be enriched in PCNSL and has been demonstrated to occur in between 38% to 50% of cases (36,37). In addition, CD79B, a component of the B-cell receptor signaling pathway, is mutated in an approximately 20% of cases, providing evidence that dysregulation of the B-cell receptor and NFκB to the pathogenesis of PCNSL (38). Silencing of the cell cycle regulator CDKN2A occurs in 50% of CNS lymphomas and is linked to inferior outcome (34,36).
The molecular basis for selective tropism and dissemination of lymphoma within the brain are biological questions that are fundamental to the pathogenesis of PCNSL. Expression of chemokines CXCL12 (SDF-1) and CXCL-13 (B-lymphocyte chemoattractant) within PCNSL tumors has been demonstrated (39-41) and chemotactic responsiveness to these molecules by CNS lymphoma tumor cells recently demonstrated, providing evidence for their role as neurotropic factors. Moreover, high CXCL-13 concentration in tumor-associated CSF correlates with adverse prognosis, supporting its role as a pro-survival factor in PCNSL. In addition, the determination of the CSF concentration of CXCL-13, as well as IL-10, may be useful in facilitating diagnosis of CNS lymphoma; bivariate upregulated expression of each protein in CSF has diagnostic sensitivity at least two-fold greater than flow-cytometry and cytology. In a multicenter study that involved discovery and validation cohorts, the positive predictive value of bivariate elevation of IL-10 plus CXCL-13 in CSF was determined to be 95% in the identification of newly diagnosed HIV-negative PCNSL (42).
A number of studies have provided evidence that the JAK/STAT signaling pathway mediates pro-survival signals in PCNSL. Interleukin-4, a B-cell growth factor that mediates intracellular signals via JAK/STAT, is upregulated at the transcript and protein level within the vascular microenvironment in PCNSL tumors (22). Increased concentration of IL-10 (another first messenger in JAK/STAT signaling) is upregulated in the vitreous and CSF in PCNSL and, in independent studies, correlated with adverse prognosis (43,44). Finally, intratumoural JAK1 transcripts are upregulated and JAK1 activation in PCNSL has been confirmed (22,45). Elevated IL-10 expression plus activation of JAK/STAT signaling are consistent with aberrant activation of the MyD88 pathway in PCNSL (46) (Figure 2).
While under physiologic conditions, the brain is largely assumed to be an immunologically quiescent or “privileged site”, histopathologic evaluation of diagnostic specimens of PCNSL demonstrates a robust inflammatory response, with infiltrating reactive T-cells and activated macrophages. Of note, reactive, perivascular T-cell infiltrates in PCNSL may be predictive of a favorable outcome, supporting the development of immunotherapies that potentiate T-cell-mediated immune surveillance (47).
Clinical presentation
Median age of PCNSL at diagnosis is 56 years with a male-to-female ratio of 1.2:1-1.7:1. Clinical presentation usually reflects the neuroanatomical location of the lesion(s). Greater than 60% of PCNSL patients present with cognitive, motor or constitutional symptoms; 20% with seizures(48). Leptomeningeal disease is confirmed in 15-20% (49). Thirty percent of PCNSL patients note visual symptoms that may be attributed to IOL, heralded by non-specific symptoms of blurred vision, decreased acuity, floaters, eye pain, and photophobia, typically with binocular involvement. At presentation, the pace of neurologic decline is variable; some patients complain of chronic visual symptoms that antedate the diagnosis by years, while for others, disease progression is aggressive. Notably, in a recent series of patients with rapid neurological decline who presented for diagnostic brain biopsy, the most frequent diagnosis was PCNSL (48).
Diagnostic and staging evaluation of the patient with neurological symptoms
Because patients with PCNSL/IOL commonly present with a variety of nonspecific and/or subtle neurologic symptoms, establishing the diagnosis may be protracted and it is not uncommon for the interval between first onset of disease signs to extend months-to-years before a diagnosis is established. The cornerstone of diagnostic testing in suspected PCNSL is MRI-based examination of the brain, with gadolinium contrast. In 95% of PCNSL, there is pathologic enhancement that localizes homogeneously to dominant tumor masses. Radiographic evidence of necrosis is rare and is one of the radiographic features that can help to distinguish CNS lymphoma from glioblastoma. Among the immunocompetent, lesions are solitary in 65% of PCNSL patients and multifocal in 35%. Involvement of the brain hemispheres is the most common localization (38%) followed by thalamus and basal ganglia (16%), corpus callosum and related structures (14%) periventricular loci (12%) and the cerebellum (9%) (50).
Glucocorticoids typically induce a rapid improvement in symptoms, and radiographic responses in at least 40% of patients, steroid-induced responses also increase the risk of a non-diagnostic vitreal or brain biopsy (51). Steroid-induced diagnostic delays may extend for several months, and on occasion, steroid-induced regressions of sentinel lesions may delay diagnosis of PCNSL or IOL for years (52). It is therefore important to emphasize that empiric glucocorticoids such as dexamethasone be tapered rapidly, or not administered until a diagnosis is established. If PCNSL is confirmed, steroids need to be tapered and discontinued as quickly as possible, unless there is symptomatic and/or life-threatening tumor-associated mass effect.
The standard diagnostic approach for PCNSL is stereotactic brain biopsy; in selected cases, a subtotal resection, may be appropriate if deemed to be safe. Flow-cytometric or cytologic analysis of meningeal lymphoma cells isolated from CSF or vitrectomy may also yield the diagnosis. Notably, while flow-cytometry has increased diagnostic yield relative to cytology, CSF needs to be efficiently processed for studies designed to identify, in most cases, a kappa or lambda-restricted B-cell lymphoma. In our experience, repeated CSF cytological or flow-cytometric studies infrequently improve diagnostic yield, supporting development of innovative diagnostic methods based upon proteomics or analysis of non-coding RNA’s in PCNSL (43,53,54).
Given that approximately 80% of patients with IOL will exhibit CNS dissemination, magnetic resonance imaging of the brain with gadolinium is indicated in the work-up of idiopathic uveitis in which lymphoma is a diagnostic consideration. Additional staging tests for IOL include fluorescence angiography and optical coherent as well as determination of intraocular concentrations of IL-10 and IL-6 may also be useful adjuncts to diagnosis (55).
Staging evaluation for the patient with presumptive PCNSL includes complete ophthalmologic examination including slit lamp. Systemic staging is also indicated, given that between 4-12% of patients with presumptive PCNSL manifest extra-CNS disease (56). Therefore, body imaging via contrast-enhanced computed tomography (CT) of the chest, abdomen and pelvis, as well as bone marrow biopsy are standard; the value of positron emission tomography imaging in the staging evaluation of PCNSL has not been established (57), but may nevertheless be useful in evaluation of possible concomitant testicular involvement. Serological testing for HIV, hepatitis C and B, plus measurement of serum LDH are standard baseline tests (58).
Clinical prognostic determinants in PCNSL
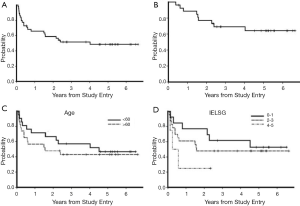
The International Extranodal Lymphoma Study Group (IELSG) identified five clinical variables that correlate with prognosis in PCNSL, three are shared with systemic NHL: elevated LDH, age greater than 60, and Eastern Cooperative Group performance status greater than 1; parameters specific to PCNSL include elevated CSF protein as well as tumor location within the deep regions of the brain (periventricular, basal ganglia, brainstem and/or cerebellum). The presence of 0-1, 2-3, or 4-5 adverse risk factors correlates with 2-year survival rates of 80%, 48% or 15% (59). Notably, age has historically been considered to be the most reliable clinical prognostic factor, however there is disagreement regarding the age cut-point at which prognosis declines. While the IELSG considered age 60 years to be the cutpoint above which prognosis declines, the Memorial Sloan-Kettering (MSK) prognostic index employs a cutpoint of age 50 (60). Notably, in CALGB 50202, which evaluated intensive immunochemotherapy followed by high-dose consolidation, without WBRT, outcomes for PCNSL patients older than 60 was similar to younger patients, a result that suggests that the optimal cutpoint for age as a prognostic variable is strongly linked to the delayed effects of treatment (10,12).
Principles of management in PCNSL
Surgery: biopsy vs. resection
Until recently, most authorities have recommended against neurosurgical resection of PCNSL, based upon the scant evidence that surgical cytoreduction provides no survival benefit compared to biopsy and increases the risk of post-operative neurologic deficit (61,62). Analysis of the results of the German PCNSL SG-1 trial provided the first evidence that aggressive resection of PCNSL at diagnosis correlated with improved progression-free survival (PFS) (63). In our experience, safe resection of lesions often provides immediate relief of mass effect, facilitates glucocorticoid taper, and theoretically eliminates drug-resistant tumor clones, without contributing to neurologic deficits, particularly when performed using modern neurosurgical mapping techniques.
Whole brain irradiation (WBRT) in PCNSL
The impact of WBRT in the treatment of PCNSL is compromised by at least three important problems: (I) inadequate local control of lymphoma; (II) dissemination of radiographically-occult lymphoma cells outside of the radiation port; (III) long-term deleterious effects of radiation on normal brain function. In one study, the use of WBRT as the sole intervention in PCNSL yielded a median survival of only 11.6 months, and greater than 60% of patients experienced lymphoma progression within the irradiated field (64). The archetypical features of late, delayed neurotoxicity caused by WBRT of PCNSL include incontinence, gait and memory disturbances-toxicities that are most evident in patients older than 60. The majority of PCNSL survivors that exhibit late-delayed neurotoxicity caused by WBRT ultimately require custodial care (65). While in preliminary studies, lower doses of WBRT were associated with neurotoxicity that is barely discernable (66). additional validation of these results are necessary, and, given the established deleterious neurocognitive effects of prophylactic cranial irradiation at 30 Gy (67), it is plausible that delayed neurotoxicity secondary to WBRT is a continuous variable in terms of its relationship to dose. Importantly, a recent update demonstrated that PCNSL patients older than 60 that are treated with low-dose WBRT (23.4 Gy) as consolidation experienced markedly inferior outcome in PFS compared to patients younger than 60 (68). Given the rising incidence in PCNSL in older patients, these results substantiate the need for innovative strategies that defer or eliminate WBRT as therapy in PCNSL.
Induction chemotherapeutic strategies in PCNSL
The feasibility and efficacy of HD-MTX in CNS lymphomas was established in the 1970’s (5,6) and led to its incorporation more broadly in induction and salvage regimens. Remarkably, use of HD-MTX has been identified in multivariate analysis as the most important treatment-related prognostic variable related to survival in CNS lymphomas (69).
Nevertheless, the optimal dose of methotrexate has not been defined. Based upon our experience it is clear that systemic doses greater than or equal to 1 gm/m2 mediate lymphocytotoxic effects within brain parenchyma, in agreement with other investigators (6). In a landmark study, Glantz and colleagues demonstrated that intravenous methotrexate administered at 8 g/m2 over four hours yielded higher cytotoxic levels of methotrexate (greater than 1 micro Molar), in serum and CSF compared to intrathecal methotrexate (12 mg) at 48 and 72 hours (70). Also, investigators at the MSK Cancer Center demonstrated that elimination of intrathecal methotrexate during initial therapy for PCNSL did not affect outcome, as long as patients received HD-MTX at doses of 3.5 gm/m2 (71). In summary, these important studies indicate that high-dose intravenous methotrexate, administered every two weeks for a minimum of six cycles, can be used to treat large cell lymphoma within brain and leptomeningeal compartments, without intrathecal therapy (10). Among the many therapeutic issues in PCNSL yet to be resolved is the question of what is the optimal number of cycles of HD-MTX administered during induction. Given the evidence that greater than four cycles of methotrexate are necessary to obtain an effective remission before dose-intensive consolidation (72), our protocol is administer eight cycles of HD-MTX during induction, assuming a complete remission (CR) is attained by cycle six. An emerging area of translational medicine that is relevant concerns recent insights into the expression and activity of efflux transporters at the blood brain barrier, such as ABCG2 and ABCC4 as these molecular pumps jointly contribute to methotrexate removal from the CNS (73).
Prevention and management of high dose methotrexate (HD-MTX) toxicity
It is important for the hematologist/oncologist to be educated in management of the potential toxicities of HD-MTX, in particular methotrexate nephropathy, caused by precipitation of methotrexate and its metabolite 7-OH-methotrexate within renal tubules. Measures to prevent this life-threatening complication include vigorous hydration, urine alkalinization, and avoidance of agents that interact with HD-MTX such as derivatives of penicillin. Third-space effusions must be drained before methotrexate administration, with serial monitoring of serum methotrexate plus leukovorin rescue started at 24 hours. Elevated serum creatinine at 48 hours may be a useful surrogate for delayed methotrexate clearance (74). Delayed methotrexate excretion mandates continued alkalinization and hydration as well as escalated leukovorin dosing. Additional interventions for delayed methotrexate clearance include carboxypeptidase-G2 (CPDG2, glucarpidase), a recombinant enzyme that reduces toxic serum methotrexate within 15 minutes, via direct hydrolysis of methotrexate (75), thus mitigating the toxic and potentially life-threatening effects of methotrexate on organ function (76).
Combined-modality regimens
Combined modality therapy for PCNSL was pioneered by DeAngelis and colleagues at MSK Cancer Center and consisted of HD-MTX plus procarbazine and vincristine, followed by WBRT and high-dose cytarabine. Evaluation of this approach in a multicenter RTOG trial demonstrated a median PFS of 24 months (77) (Table 1). Because of this encouraging efficacy, combined-modality therapy became a widely adopted approach for PCNSL (78,79). In a multicenter randomized phase II study, Ferreri and colleagues evaluated HD-MTX-based induction, minus or plus high-dose cytarabine followed by consolidative WBRT: the median failure-free survival in patients receiving HD-Ara-C in combination with HD-MTX was eight months; in contrast, the median failure-free survival of patients treated with HD-MTX without cytarabine, was only four months (80). In the SG-1 randomized trial involving 551 PCNSL patients in which half the subjects received WBRT as first-line consolidation, Thiel and colleagues provided strong evidence that omission of WBRT from first-line chemotherapy did not impact survival. While the investigators could demonstrate a modestly favorable effect of WBRT on PFS after methotrexate-based induction, this did not translate into improved overall survival, likely to a significant degree, attributable to the severe neurotoxicity detected in nearly half of patients in the radiotherapy arm (81).

Full table
Dose-intensive chemotherapy consolidation
Given the recognition of the inadequate efficacy as well as severe neurotoxicity associated with WBRT, there has been significant interest in the role of high-dose chemotherapeutic consolidation, including autologous stem cell rescue, in the treatment of PCNSL, at diagnosis as well as in the relapsed setting. Regimens that contain CNS penetrant agents such as carmustine, thiotepa, cyclophosphamide, busulfan, high-dose cytarabine and etoposide are associated with the best results (7,9,82,83) (Table 2). In one study, results obtained using the BEAM combination (carmustine, etoposide, cytarabine and melphalan) followed by autologous stem cell rescue did not appear promising, however in this trial a significant proportion of patients had unsatisfactory disease control before receipt of myeloablative therapy, possibly because of the abbreviated induction used in this trial (72).

Full table
In a French study, Soussain and colleagues evaluated dose-intensive chemotherapy and autologous stem cell transplant in recurrent CNS lymphomas and IOL. These investigators also noted that the drug combination of high-dose cytarabine plus etoposide constituted a highly potent salvage regimen for relapsed/refractory CNS lymphomas: 12 of 14 patients attained responses, eight of these were complete responses (82). Responding patients received a myeloablative regimen consisting of thiotepa, busulfan and cyclophosphamide followed by stem cell rescue.
Beginning in 2001, investigators at the University of California, San Francisco (UCSF), began to evaluate dose-intensive chemotherapy as first-line consolidation, without WBRT, after induction immunochemotherapy in newly-diagnosed PCNSL. We developed a two-step regimen: the induction phase uses HD-MTX given every two weeks with oral temozolomide and rituximab (MT-R). Methotrexate is administered at 8 g/m2 with appropriate dose reductions and leucovorin rescue day 2. Intravenous rituximab is administered starting day 3, and weekly for six infusions, an interval during which the blood-brain barrier may be most compromised by angiotropic lymphoma (86). A recent retrospective study also supports the incorporation of rituximab with HD-MTX in the treatment of newly-diagnosed patients with PCNSL in that patients in this series that were treated with high dose methotrexate plus rituximab had a markedly superior rate of CR and PFS compared to patients treated with HD-MTX as monotherapy (CR rate 73% vs. 36%; median PFS 4.5 vs. 26.7 months) (87). Temozolomide is a lipophilic alkylating agent that has activity at relapse in CNS lymphoma, alone and in combination with rituximab (88-90). Importantly, temozolomide is associated with a superior health-related quality of life and toxicity characteristics compared to procarbazine (91,92). Temozolomide is administered on a monthly schedule in a five-day course, beginning days 7-11. To consolidate response after induction MT-R, PCNSL patients received intensive consolidation with non-cross-resistant agents with the EA combination: 96-hour infusional etoposide plus eight doses of cytarabine at 2 gm/m2 (93-95). Notably, infusional etoposide is incorporated within the EPOCH regimen (etoposide, doxorubicin, cyclophosphamide, vincristine and prednisone), which is active against large B-cell lymphoma (96,97). A number of studies provide evidence for the activity of etoposide in brain tumours, including CNS lymphoid leukemia (98). Notably, when given in combination with CHOP in patients with aggressive lymphoma, etoposide was associated with a reduced risk of secondary CNS lymphoma (99).
A key goal of the two-step MT-R EA program was to develop an induction regimen that incorporates an alkylator, temozolomide (89) as well as rituximab (100), and yet causes minimal myelosuppression, to enable minimal treatment delays during the first weeks of treatment, the interval at which maximal lymphoma cytoreduction is achieved. Long-term follow-up of the first cohort of PCNSL patients that were treated with this regimen demonstrates that combination EA is highly effective as consolidation after MT-R in newly diagnosed PCNSL (10). Of the first fourteen PCNSL patients that received MT-R followed by EA consolidation, twelve remain in remission, with a median follow-up of greater than 72 months. Based on encouraging institutional phase I data, the MT-R plus EA regimen was evaluated in CALGB 50202, which provided the first evidence for the multicenter feasibility of high-dose chemotherapy in newly-diagnosed PCNSL. The rate of complete response to MT-R induction in CALGB 50202 was 66% and the 2-year PFS was 59%, which thus far has not been surpassed in a multicenter study involving chemotherapy without brain radiotherapy in PCNSL. Moreover, the median time to progression of all 50202 patients, 4 years, is two times longer than achieved with combined-modality therapy in multicenter trials using standard-dose WBRT and appears to compare favorably to reduced-dose WBRT (77,81). Other key results of the CALGB 50202 study include the fact that outcomes for PCNSL patients were similar for patients older than 60 compared to younger patients (12) (Table 1, Figure 3) and the observation that the PFS curves reached a stable plateau, supporting the hypothesis that long-term survival can be achieved in PCNSL without whole brain radiotherapy.
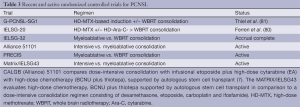
Based upon the encouraging results of CALGB 50202, a successor randomized phase II trial, CALGB 51101, has been activated in the United States and endorsed by the major cooperative groups: Alliance, ECOG and SWOG. In CALGB 51101, after upfront randomization and remission induction therapy with MT-R, patients receive either consolidation with EA or myeloablative therapy and stem cell transplant with carmustine plus thiotepa (7). Like CALGB 51101, the European MATRIX/IELSG43 randomized trial is also comparing high-dose consolidation chemotherapy with conventional consolidative chemotherapy (dexamethasone, VP16, ifosfamide and carboplatin) (Table 3).
Full table
Neurocognitive function
Given the recent progress in outcomes in PCNSL, the issue of treatment-related neurotoxicity among survivors merits evaluation. While there is evidence that reduced-dose brain radiotherapy may be associated with milder cognitive dysfunction among PCNSL survivors compared to standard-dose WBRT (66), reduced doses of WBRT as consolidation are associated with impairments of verbal memory and motor speed. By contrast, PCNSL patients treated with HD-MTX without consolidative WBRT do not appear to exhibit comparably severe cognitive dysfunction as determined by post-treatment neuropsychological testing; nevertheless PCNSL patients treated with HD-MTX without WBRT nevertheless score lower than normative control subjects in evaluations of motor speed, selective attention, executive function, delayed recall and verbal learning (101). Given that PCNSL is a highly-infiltrative brain tumor that is associated with a spectrum of neurologic symptoms, the determination of whether impairments of neurologic function are caused by lymphoma or are the consequence of delayed neurotoxicity of agents such as methotrexate remains a major challenge.
Treatment of recurrent CNS lymphomas
Several studies from Europe have demonstrated that dose-intensive chemotherapy with autologous stem cell transplant is an attractive option in the management of relapsed CNS and IOL (8,82,84). Recently, the Berlin group presented their experience using a salvage regimen consisting of HD-MTX-based chemotherapy in combination with other CNS-penetrant agents, ifosfamide, thiotepa, cytarabine and depocyt, followed by myeloablative therapy (carmustine, etoposide, thiotepa) and stem cell transplant. This approach yielded a 2-year PFS rate of 49% (9). A key consideration in treatment of recurrent CNS lymphomas is whether the lymphoma is methotrexate-sensitive. In the setting of recurrent disease that is methotrexate-sensitive, our approach is to administer additional cycles of HD-MTX, to achieve maximal cytoreduction, (six-to-eight cycles), followed by dose-intensive chemotherapy consolidation using non cross-resistant, CNS penetrant agents such as thiotepa (9,85,102). High-dose carmustine-based therapy without thiotepa has also been studied (83) (Table 1).
For CNS lymphomas that have progressed within six months of dose-intensive chemotherapeutic consolidation, second-line high-dose salvage may not be a reasonable option. Such patients may be managed with additional HD-MTX, pemetrexed (103) WBRT or investigational agents.
Rituximab in CNS lymphomas
Because the blood-brain barrier excludes molecules that exceed 400 daltons, it is not surprising that most studies report that less than 1% of systemic rituximab penetrates the leptomeningeal space (104). While rituximab has become a cornerstone of therapy in systemic B-cell NHL, a number of studies demonstrated that the addition of rituximab to CHOP may not significantly decrease the rate of CNS recurrence of systemic large B-cell lymphoma compared to CHOP alone (105-107). Nevertheless, intravenous rituximab can induce responses of contrast-enhancing lesions in CNS lymphoma, likely in lesions in which there is substantial disruption of the blood-brain barrier (100).
Intraventricular rituximab in CNS Lymphomas
We recently evaluated the safety and efficacy of intraventricular rituximab, both as monotherapy and in combination with intraventricular methotrexate in the setting of two phase I multicenter trials involving patients with recurrent CNS lymphomas. These studies demonstrated that, when diluted in preservative-free normal saline and administered into ventricular CSF, 10 and 25 mg doses of rituximab are well-tolerated and elicit responses within leptomeninges, intraocular compartments and in small parenchymal lesions. The efficacy of intraventricular rituximab was additive or synergistic with methotrexate. One of the key findings was that intraventricular rituximab/methotrexate was particularly active in patients with a high burden of leptomeningeal lymphoma. These studies also suggested that intraventricular rituximab overcomes the problem of the blood-brain barrier, in that CSF responses were documented in patients with baseline serum rituximab concentrations greater than 15 g/mL. Notably, two patients achieved a first complete response of CNS lymphoma with intraventricular rituximab/methotrexate, including one with CNS lymphoma refractory to high-dose systemic and intrathecal methotrexate plus 20 previous intravenous rituximab infusions (108,109). An important mechanistic explanation for the rapid efficacy of intraventricular rituximab is provided by the recent demonstration of activation of the complement cascade at the level of C3 as well as the C5b-9 membrane attack complex within CSF upon intra-CSF rituximab administration, as well as pharmacokinetic that that suggests penetration of rituximab into deep neural tissue (110) (Figure 4).
Given the evidence for activity of rituximab in CNS lymphomas, as monotherapy and in combination with methotrexate-based induction (111), a number of protocols now incorporate this anti-CD20 monoclonal antibody as a component of induction therapy in PCNSL. While several studies demonstrate its activity at relapse, intraventricular rituximab remains investigational and the combination of intraventricular plus intravenous rituximab with oral lenalidomide for recurrent CNS lymphomas is currently being studied in phase I investigation (NCT01542918).
Treatment of intraocular lymphoma (IOL)
Most cases of IOL involve large B-cell NHL, and are classified as either primary vitreoretinal lymphoma (PVRL) or uveal lymphomas; these are divided into primary neoplasms of the choroid, iris and ciliary body, or secondary choroidal lymphomas in patients with disseminated systemic NHL. Importantly, between 65% to 90% of patients with PVRL ultimately disseminate throughout the neuroaxis, usually within 30 months. Conversely, IOL impacts between 15-25% of patients with PCNSL.
Therapies for PVRL can be divided into local approaches such as ocular radiation or intravitreal therapy vs. systemic chemotherapy. External beam radiotherapy using opposed lateral beams to the eyes, is well tolerated, and yields low rates of local recurrence. Common complications of ocular radiotherapy are mild and include dry eye, cataracts and mild radiation retinopathy (112). Intravitreal methotrexate and rituximab may be useful in the management of unilateral disease or prior ocular radiation (113,114). Treatment-related complications of intravitreal methotrexate include vitreous hemorrhage, endophthalmitis, retinal detachment and hypotony (115). Systemic therapeutic options for IOL include HD-MTX (116), high-dose cytarabine and trofasfamide (117). Notably, in PVRL, the administration of HD-MTX plus binocular irradiation provides local disease control and also addresses the probability of subclinical disease throughout the neuroaxis (118). Our approach to patients with primary IOL and/or concomitant PCNSL with IOL usually involves three steps: (I) HD-MTX--based induction (MT-R, if the disease is CD20+); (II) dose-intensive consolidation as used in CALGB 50202 (EA); (III) binocular but not WBRT if there is persistence and/or recurrence of isolated IOL after completion of dose-intensive chemotherapy consolidation.
PCNSL in the immunocompromised host
While HIV-associated PCNSL declined in incidence with advent of highly-active antiretroviral therapy (HAART), PCNSL continues to be a significant AIDS-defining illness that can represent a major therapeutic challenge. Feasibility and efficacy of HD-MTX in HIV-associated PCNSL has been demonstrated (119). Similarly, in the setting of CNS PTLD, reconstitution of immune function is a first principle in management, and can be achieved by reduction in immunosuppression. HD-MTX may be effective but its implementation needs to be balanced with risk of allograft failure (120). Rituximab is also active in the CNS complications of PTLD, via intravenous as well as intrathecal administration (121).
Conclusions and future directions
Over the course of the past half-century, the hematology/oncology community has made significant progress in the treatment of PCNSL, an aggressive variant of large B-cell lymphoma. We can now anticipate that between 40-50% of PCNSL patients will exhibit long-term survival and a significant proportion may be cured. It is likely that the next five years of clinical trials will focus on optimization of interventions based upon high-dose chemotherapy.
At least 40-50% of PCNSL patients will develop disease refractory to the established armamentarium of agents, it is now imperative that additional studies explore the potential efficacy of selective agents that target candidate resistance mechanisms in high-risk PCNSL patients. For example, pharmacologic agents that evaluate disruption of pathways involving the B-cell receptor, JAK-STAT, toll-like receptor, mTOR, and PIM kinases should be considered high priority in early phase investigation in PCNSL. Another key target is MUM-1/IRF-4, targeted by the IMiD category of small molecule agents such as lenalidomide or pomalidomide, currently under evaluation in PCNSL in early phase clinical trials (122). Elucidation of a molecular prognostic index for risk-adapted therapies in PCNSL is also a key goal for research in this field. Transformative advances are required in PCNSL given its predilection for an aging population that cannot tolerate dose-intensive chemotherapy or WBRT.
Acknowledgements
Funding: James L. Rubenstein receives research funding from Celgene and Genentech for a phase I clinical trial. This work was supported by the National Institutes of Health, University of California San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (P30 AI027763), NIH R01CA139-83-01A1, and by the Leukemia & Lymphoma Society (JLR).
Disclosure: The authors declare no conflict of interest.
References
- Villano JL, Koshy M, Shaikh H, et al. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer 2011;105:1414-8. [PubMed]
- Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg 1988;68:835-53. [PubMed]
- Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol 2006;24:1281-8. [PubMed]
- Norden AD, Drappatz J, Wen PY, et al. Survival among patients with primary central nervous system lymphoma, 1973-2004. J Neurooncol 2011;101:487-93. [PubMed]
- Ervin T, Canellos GP. Successful treatment of recurrent primary central nervous system lymphoma with high-dose methotrexate. Cancer 1980;45:1556-7. [PubMed]
- Skarin AT, Zuckerman KS, Pitman SW, et al. High-dose methotrexate with folinic acid in the treatment of advanced non-Hodgkin lymphoma including CNS involvement. Blood 1977;50:1039-47. [PubMed]
- Illerhaus G, Müller F, Feuerhake F, et al. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica 2008;93:147-8. [PubMed]
- Bromberg JE, Doorduijn JK, Illerhaus G, et al. Central nervous system recurrence of systemic lymphoma in the era of stem cell transplantation--an International Primary Central Nervous System Lymphoma Study Group project. Haematologica 2013;98:808-13. [PubMed]
- Korfel A, Elter T, Thiel E, et al. Phase II study of central nervous system (CNS)-directed chemotherapy including high-dose chemotherapy with autologous stem cell transplantation for CNS relapse of aggressive lymphomas. Haematologica 2013;98:364-70. [PubMed]
- Wieduwilt MJ, Valles F, Issa S, et al. Immunochemotherapy with intensive consolidation for primary CNS lymphoma: a pilot study and prognostic assessment by diffusion-weighted MRI. Clin Cancer Res 2012;18:1146-55. [PubMed]
- Rubenstein JL, Gupta NK, Mannis GN, et al. How I treat CNS lymphomas. Blood 2013;122:2318-30. [PubMed]
- Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol 2013;31:3061-8. [PubMed]
- Cingolani A, Gastaldi R, Fassone L, et al. Epstein-Barr virus infection is predictive of CNS involvement in systemic AIDS-related non-Hodgkin’s lymphomas. J Clin Oncol 2000;18:3325-30. [PubMed]
- Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol 1999;43:199-201. [PubMed]
- Camilleri-Broët S, Crinière E, Broët P, et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood 2006;107:190-6. [PubMed]
- Lai R, Rosenblum MK, DeAngelis LM. Primary CNS lymphoma: a whole-brain disease? Neurology 2002;59:1557-62. [PubMed]
- Fine HA, Mayer RJ. Primary central nervous system lymphoma. Ann Intern Med 1993;119:1093-104. [PubMed]
- Rubenstein JL, Treseler P, O’Brien JM. Pathology and genetics of primary central nervous system and intraocular lymphoma. Hematol Oncol Clin North Am 2005;19:705-17. [PubMed]
- Shenkier TN, Blay JY, O’Neill BP, et al. Primary CNS lymphoma of T-cell origin: a descriptive analysis from the international primary CNS lymphoma collaborative group. J Clin Oncol 2005;23:2233-9. [PubMed]
- Tu PH, Giannini C, Judkins AR, et al. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: a primary low-grade CNS lymphoma that mimics meningioma. J Clin Oncol 2005;23:5718-27. [PubMed]
- Braaten KM, Betensky RA, de Leval L, et al. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res 2003;9:1063-9. [PubMed]
- Rubenstein JL, Fridlyand J, Shen A, et al. Gene expression and angiotropism in primary CNS lymphoma. Blood 2006;107:3716-23. [PubMed]
- Fischer L, Hummel M, Korfel A, et al. Differential micro-RNA expression in primary CNS and nodal diffuse large B-cell lymphomas. Neuro Oncol 2011;13:1090-8. [PubMed]
- Cady FM, O’Neill BP, Law ME, et al. Del(6)(q22) and BCL6 rearrangements in primary CNS lymphoma are indicators of an aggressive clinical course. J Clin Oncol 2008;26:4814-9. [PubMed]
- Jordanova ES, Riemersma SA, Philippo K, et al. Hemizygous deletions in the HLA region account for loss of heterozygosity in the majority of diffuse large B-cell lymphomas of the testis and the central nervous system. Genes Chromosomes Cancer 2002;35:38-48. [PubMed]
- Tun HW, Personett D, Baskerville KA, et al. Pathway analysis of primary central nervous system lymphoma. Blood 2008;111:3200-10. [PubMed]
- Booman M, Szuhai K, Rosenwald A, et al. Genomic alterations and gene expression in primary diffuse large B-cell lymphomas of immune-privileged sites: the importance of apoptosis and immunomodulatory pathways. J Pathol 2008;216:209-17. [PubMed]
- Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117:5019-32. [PubMed]
- Harada K, Nishizaki T, Kubota H, et al. Distinct primary central nervous system lymphoma defined by comparative genomic hybridization and laser scanning cytometry. Cancer Genet Cytogenet 2001;125:147-50. [PubMed]
- Boonstra R, Koning A, Mastik M, et al. Analysis of chromosomal copy number changes and oncoprotein expression in primary central nervous system lymphomas: frequent loss of chromosome arm 6q. Virchows Arch 2003;443:164-9. [PubMed]
- Courts C, Montesinos-Rongen M, Brunn A, et al. Recurrent inactivation of the PRDM1 gene in primary central nervous system lymphoma. J Neuropathol Exp Neurol 2008;67:720-7. [PubMed]
- Nakamura M, Kishi M, Sakaki T, et al. Novel tumor suppressor loci on 6q22-23 in primary central nervous system lymphomas. Cancer Res 2003;63:737-41. [PubMed]
- Braggio E, McPhail ER, Macon W, et al. Primary central nervous system lymphomas: a validation study of array-based comparative genomic hybridization in formalin-fixed paraffin-embedded tumor specimens. Clin Cancer Res 2011;17:4245-53. [PubMed]
- Schwindt H, Vater I, Kreuz M, et al. Chromosomal imbalances and partial uniparental disomies in primary central nervous system lymphoma. Leukemia 2009;23:1875-84. [PubMed]
- Montesinos-Rongen M, Schmitz R, Brunn A, et al. Mutations of CARD11 but not TNFAIP3 may activate the NF-kappaB pathway in primary CNS lymphoma. Acta Neuropathol 2010;120:529-35. [PubMed]
- Gonzalez-Aguilar A, Idbaih A, Boisselier B, et al. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res 2012;18:5203-11. [PubMed]
- Montesinos-Rongen M, Godlewska E, Brunn A, et al. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol 2011;122:791-2. [PubMed]
- Montesinos-Rongen M, Schäfer E, Siebert R, et al. Genes regulating the B cell receptor pathway are recurrently mutated in primary central nervous system lymphoma. Acta Neuropathol 2012;124:905-6. [PubMed]
- Fischer L, Korfel A, Pfeiffer S, et al. CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin Cancer Res 2009;15:5968-73. [PubMed]
- Smith JR, Braziel RM, Paoletti S, et al. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood 2003;101:815-21. [PubMed]
- Smith JR, Falkenhagen KM, Coupland SE, et al. Malignant B cells from patients with primary central nervous system lymphoma express stromal cell-derived factor-1. Am J Clin Pathol 2007;127:633-41. [PubMed]
- Rubenstein JL, Wong VS, Kadoch C, et al. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood 2013;121:4740-8. [PubMed]
- Roy S, Josephson SA, Fridlyand J, et al. Protein biomarker identification in the CSF of patients with CNS lymphoma. J Clin Oncol 2008;26:96-105. [PubMed]
- Sasayama T, Nakamizo S, Nishihara M, et al. Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro Oncol 2012;14:368-80. [PubMed]
- Sung CO, Kim SC, Karnan S, et al. Genomic profiling combined with gene expression profiling in primary central nervous system lymphoma. Blood 2011;117:1291-300. [PubMed]
- Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011;470:115-9. [PubMed]
- Ponzoni M, Berger F, Chassagne-Clement C, et al. Reactive perivascular T-cell infiltrate predicts survival in primary central nervous system B-cell lymphomas. Br J Haematol 2007;138:316-23. [PubMed]
- Josephson SA, Papanastassiou AM, Berger MS, et al. The diagnostic utility of brain biopsy procedures in patients with rapidly deteriorating neurological conditions or dementia. J Neurosurg 2007;106:72-5. [PubMed]
- Fischer L, Martus P, Weller M, et al. Meningeal dissemination in primary CNS lymphoma: prospective evaluation of 282 patients. Neurology 2008;71:1102-8. [PubMed]
- Küker W, Nägele T, Korfel A, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol 2005;72:169-77. [PubMed]
- Porter AB, Giannini C, Kaufmann T, et al. Primary central nervous system lymphoma can be histologically diagnosed after previous corticosteroid use: a pilot study to determine whether corticosteroids prevent the diagnosis of primary central nervous system lymphoma. Ann Neurol 2008;63:662-7. [PubMed]
- Pirotte B, Levivier M, Goldman S, et al. Glucocorticoid-induced long-term remission in primary cerebral lymphoma: case report and review of the literature. J Neurooncol 1997;32:63-9. [PubMed]
- Rubenstein JL, Wong VS, Kadoch C, et al. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood 2013;121:4740-8. [PubMed]
- Baraniskin A, Kuhnhenn J, Schlegel U, et al. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood 2011;117:3140-6. [PubMed]
- Chan CC, Buggage RR, Nussenblatt RB. Intraocular lymphoma. Curr Opin Ophthalmol 2002;13:411-8. [PubMed]
- Ferreri AJ, Reni M, Zoldan MC, et al. Importance of complete staging in non-Hodgkin's lymphoma presenting as a cerebral mass lesion. Cancer 1996;77:827-33. [PubMed]
- Mohile NA, Deangelis LM, Abrey LE. The utility of body FDG PET in staging primary central nervous system lymphoma. Neuro Oncol 2008;10:223-8. [PubMed]
- Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23:5034-43. [PubMed]
- Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol 2003;21:266-72. [PubMed]
- Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 2006;24:5711-5. [PubMed]
- DeAngelis LM, Yahalom J, Heinemann MH, et al. Primary CNS lymphoma: combined treatment with chemotherapy and radiotherapy. Neurology 1990;40:80-6. [PubMed]
- Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg 2000;92:261-6. [PubMed]
- Weller M, Martus P, Roth P, et al. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro Oncol 2012;14:1481-4. [PubMed]
- Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys 1992;23:9-17. [PubMed]
- Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol 1998;16:859-63. [PubMed]
- Correa DD, Rocco-Donovan M, DeAngelis LM, et al. Prospective cognitive follow-up in primary CNS lymphoma patients treated with chemotherapy and reduced-dose radiotherapy. J Neurooncol 2009;91:315-21. [PubMed]
- Sun A, Bae K, Gore EM, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol 2011;29:279-86. [PubMed]
- Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 2013;31:3971-9. [PubMed]
- Blay JY, Conroy T, Chevreau C, et al. High-dose methotrexate for the treatment of primary cerebral lymphomas: analysis of survival and late neurologic toxicity in a retrospective series. J Clin Oncol 1998;16:864-71. [PubMed]
- Glantz MJ, Cole BF, Recht L, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol 1998;16:1561-7. [PubMed]
- Khan RB, Shi W, Thaler HT, et al. Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol 2002;58:175-8. [PubMed]
- Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol 2003;21:4151-6. [PubMed]
- Sane R, Wu SP, Zhang R, et al. The effect of ABCG2 and ABCC4 on the pharmacokinetics of methotrexate in the brain. Drug Metab Dispos 2014;42:537-40. [PubMed]
- Xu WQ, Zhang LY, Chen XY, et al. Serum creatinine and creatinine clearance for predicting plasma methotrexate concentrations after high-dose methotrexate chemotherapy for the treatment for childhood lymphoblastic malignancies. Cancer Chemother Pharmacol 2014;73:79-86. [PubMed]
- Green JM. Glucarpidase to combat toxic levels of methotrexate in patients. Ther Clin Risk Manag 2012;8:403-13. [PubMed]
- Fermiano M, Bergsbaken J, Kolesar JM. Glucarpidase for the management of elevated methotrexate levels in patients with impaired renal function. Am J Health Syst Pharm 2014;71:793-8. [PubMed]
- DeAngelis LM, Seiferheld W, Schold SC, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol 2002;20:4643-8. [PubMed]
- DeAngelis LM, Yahalom J, Thaler HT, et al. Combined modality therapy for primary CNS lymphoma. J Clin Oncol 1992;10:635-43. [PubMed]
- Glass J, Gruber ML, Cher L, et al. Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: long-term outcome. J Neurosurg 1994;81:188-95. [PubMed]
- Ferreri AJ, Reni M, Foppoli M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 2009;374:1512-20. [PubMed]
- Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol 2010;11:1036-47. [PubMed]
- Soussain C, Suzan F, Hoang-Xuan K, et al. Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol 2001;19:742-9. [PubMed]
- Alvarnas JC, Negrin RS, Horning SJ, et al. High-dose therapy with hematopoietic cell transplantation for patients with central nervous system involvement by non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant 2000;6:352-8. [PubMed]
- Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: SociéociFrançaise de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol 2008;26:2512-8. [PubMed]
- Cote GM, Hochberg EP, Muzikansky A, et al. Autologous stem cell transplantation with thiotepa, busulfan, and cyclophosphamide (TBC) conditioning in patients with CNS involvement by non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2012;18:76-83. [PubMed]
- Ott RJ, Brada M, Flower MA, et al. Measurements of blood-brain barrier permeability in patients undergoing radiotherapy and chemotherapy for primary cerebral lymphoma. Eur J Cancer 1991;27:1356-61. [PubMed]
- Holdhoff M, Ambady P, Abdelaziz A, et al. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology 2014;83:235-9. [PubMed]
- Reni M, Ferreri AJ, Landoni C, et al. Salvage therapy with temozolomide in an immunocompetent patient with primary brain lymphoma. J Natl Cancer Inst 2000;92:575-6. [PubMed]
- Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer 2007;96:864-7. [PubMed]
- Wong ET, Tishler R, Barron L, et al. Immunochemotherapy with rituximab and temozolomide for central nervous system lymphomas. Cancer 2004;101:139-45. [PubMed]
- Osoba D, Brada M, Yung WK, et al. Health-related quality of life in patients with anaplastic astrocytoma during treatment with temozolomide. Eur J Cancer 2000;36:1788-95. [PubMed]
- Osoba D, Brada M, Yung WK, et al. Health-related quality of life in patients treated with temozolomide versus procarbazine for recurrent glioblastoma multiforme. J Clin Oncol 2000;18:1481-91. [PubMed]
- Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol 2009;27:6101-8. [PubMed]
- Damon L, Damon LE, Gaensler K, et al. Impact of intensive PBSC mobilization therapy on outcomes following auto-SCT for non-Hodgkin's lymphoma. Bone Marrow Transplant 2008;42:649-57. [PubMed]
- Linker CA, Owzar K, Powell B, et al. Auto-SCT for AML in second remission: CALGB study 9620. Bone Marrow Transplant 2009;44:353-9. [PubMed]
- Wilson WH, Bryant G, Bates S, et al. EPOCH chemotherapy: toxicity and efficacy in relapsed and refractory non-Hodgkin’s lymphoma. J Clin Oncol 1993;11:1573-82. [PubMed]
- Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol 2008;26:2717-24. [PubMed]
- Relling MV, Mahmoud HH, Pui CH, et al. Etoposide achieves potentially cytotoxic concentrations in CSF of children with acute lymphoblastic leukemia. J Clin Oncol 1996;14:399-404. [PubMed]
- Boehme V, Zeynalova S, Kloess M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma--a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Ann Oncol 2007;18:149-57. [PubMed]
- Batchelor TT, Grossman SA, Mikkelsen T, et al. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology 2011;76:929-30. [PubMed]
- Correa DD, Shi W, Abrey LE, et al. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro Oncol 2012;14:101-8. [PubMed]
- Falzetti F, Di Ianni M, Ballanti S, et al. High-dose thiotepa, etoposide and carboplatin as conditioning regimen for autologous stem cell transplantation in patients with high-risk non-Hodgkin lymphoma. Clin Exp Med 2012;12:165-71. [PubMed]
- Raizer JJ, Rademaker A, Evens AM, et al. Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer 2012;118:3743-8. [PubMed]
- Rubenstein JL, Combs D, Rosenberg J, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood 2003;101:466-8. [PubMed]
- Feugier P, Virion JM, Tilly H, et al. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Ann Oncol 2004;15:129-33. [PubMed]
- Tai WM, Chung J, Tang PL, et al. Central nervous system (CNS) relapse in diffuse large B cell lymphoma (DLBCL): pre- and post-rituximab. Ann Hematol 2011;90:809-18. [PubMed]
- Yamamoto W, Tomita N, Watanabe R, et al. Central nervous system involvement in diffuse large B-cell lymphoma. Eur J Haematol 2010;85:6-10. [PubMed]
- Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 2007;25:1350-6. [PubMed]
- Rubenstein JL, Li J, Chen L, et al. Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood 2013;121:745-51. [PubMed]
- Kadoch C, Li J, Wong VS, et al. Complement activation and intraventricular rituximab distribution in recurrent central nervous system lymphoma. Clin Cancer Res 2014;20:1029-41. [PubMed]
- Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol 2007;25:4730-5. [PubMed]
- Berenbom A, Davila RM, Lin HS, et al. Treatment outcomes for primary intraocular lymphoma: implications for external beam radiotherapy. Eye (Lond) 2007;21:1198-201. [PubMed]
- Kitzmann AS, Pulido JS, Mohney BG, et al. Intraocular use of rituximab. Eye (Lond) 2007;21:1524-7. [PubMed]
- Itty S, Pulido JS. Rituximab for intraocular lymphoma. Retina 2009;29:129-32. [PubMed]
- Chan CC, Rubenstein JL, Coupland SE, et al. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist 2011;16:1589-99. [PubMed]
- Batchelor TT, Kolak G, Ciordia R, et al. High-dose methotrexate for intraocular lymphoma. Clin Cancer Res 2003;9:711-5. [PubMed]
- Jahnke K, Thiel E, Bechrakis NE, et al. Ifosfamide or trofosfamide in patients with intraocular lymphoma. J Neurooncol 2009;93:213-7. [PubMed]
- Stefanovic A, Davis J, Murray T, et al. Treatment of isolated primary intraocular lymphoma with high-dose methotrexate-based chemotherapy and binocular radiation therapy: a single-institution experience. Br J Haematol 2010;151:103-6. [PubMed]
- Jacomet C, Girard PM, Lebrette MG, et al. Intravenous methotrexate for primary central nervous system non-Hodgkin’s lymphoma in AIDS. AIDS 1997;11:1725-30. [PubMed]
- Elstrom RL, Andreadis C, Aqui NA, et al. Treatment of PTLD with rituximab or chemotherapy. Am J Transplant 2006;6:569-76. [PubMed]
- van de Glind G, de Graaf S, Klein C, et al. Intrathecal rituximab treatment for pediatric post-transplant lymphoproliferative disorder of the central nervous system. Pediatr Blood Cancer 2008;50:886-8. [PubMed]
- Ponzoni M, Issa S, Batchelor TT, et al. Beyond high-dose methotrexate and brain radiotherapy: novel targets and agents for primary CNS lymphoma. Ann Oncol 2014;25:316-22. [PubMed]


