Treatment algorithm of metastatic mucosal melanoma
Introduction
Melanoma is one of the most aggressive types of cancer. Patients with advanced melanoma have a very poor prognosis. Multiple trials with chemotherapies, immunotherapies, and combined biochemotherapy regimens have failed to significantly improve outcomes in this disease. However, studies reported in recent years have revealed many breakthroughs in melanoma field. The treatment of melanoma has stepped into a new era based on an improved understanding of the molecular causes and heterogeneity of this disease. Targeted therapies and checkpoints immunotherapies have been proven successful in melanoma therapy, and some of them have been Food and Drug Administration-approved (FDA-approved). Two extraordinary advances were recently achieved when positive results from two separate studies of new therapies, ipilimumab and vemurafenib, for the treatment for advanced melanoma were published.
Mucosal melanoma accounts for less than 3% of all melanomas in Caucasian population (1). Incidences of mucosal melanoma are somewhat different in Black and Asia populations as compared to the Caucasian population, and the incidence can be up to 22.6% in Chinese melanomas (2). The biologic behavior of mucosal melanoma appears to be more aggressive than those of cutaneous origin. Interestingly, accumulating evidences suggest that clinical and pathologic features as well as the treatment outcomes are quite different between subtypes of melanoma. This chapter will review these treatments, and discuss their implications for the development of new therapeutic approaches for mucosal melanoma, a highly aggressive disease.
Chemotherapies
Metastatic melanoma is resistant to chemotherapy. Dacarbazine is the standard, FDA-approved chemotherapeutic agent for metastatic melanoma, with an overall response rate (ORR) of 13.4% and a median survival duration ranging from 5.6 to 11 months (3). Many dacarbazine-based combination regimens have been evaluated in the attempts to improve treatment outcomes. The Dartmouth regimen (dacarbazine/carmustine/cisplatin/tamoxifen), which showed a 40-50% response rate (RR) in several single-centered studies (4-6), failed to yield a survival benefit over dacarbazine monotherapy but showed more toxicities in a phase III multicenter randomized trial (7). Other than the Dartmouth regimen, agents as cisplatin/vinblastine and various cytokines were combined with dacarbazine, and none of them showed a statistical survival benefit over dacarbazine monotherapy (8-11). The efficacy of chemotherapy in mucosal melanoma is thus indefinite. In a retrospective analysis of 95 patients with metastatic malignant melanoma who had received dacarbazine-based chemotherapy in Korea, 23 patients (24.2%) had cutaneous melanoma, 37 patients (38.9%) had acral melanoma, 28 patients (29.5%) had mucosal melanoma and 7 patients had ocular melanoma. This study showed an ORR of 26.3% (cutaneous vs. mucosal: 30% vs. 20%, respectively, P=0.206) and an overall survival (OS) of 12.1 months without a significant difference in RRs between mucosal melanoma or cutaneous melanoma (12). Despite the general preconception that mucosal melanoma is refractory to systemic chemotherapy, dacarbazine-based chemotherapy seems to be a reasonable option in Asia where mucosal melanoma is more prevalent.
A research of Korean summarized the efficacy of salvage chemotherapy of paclitaxel/carboplatin (PC) for patients with metastatic mucosal melanoma after the failure of dacarbazine-based chemotherapy. They retrospectively evaluated 32 heavily pretreated patients with metastatic melanoma. Ten (31.3%) patients who received PC as salvage chemotherapy were with mucosal melanoma. All patients had been pretreated, receiving a median of three prior systemic chemotherapy regimens including dacarbazine. The median progression-free survival (PFS) was 2.53 months for all patients, 21.9% achieved PR, but no significant difference was noted between patients with mucosal and cutaneous metastatic melanoma, suggesting that PC combination chemotherapy is a reasonable therapeutic option for heavily pretreated patients with metastatic mucosal melanoma (13). New chemotherapy agents as docosahexaenoic acid (DHA)-paclitaxel also showed efficacy in metastatic mucosal melanoma patients (14).
Conclusion 1: the effects of chemotherapies are similar in cutaneous melanoma and mucosal melanoma. For both subtypes of melanoma, chemotherapies are usually associated with a poor RR.
Targeted therapies
RAS/RAF/MAPK pathway targeted therapies
The RAS/RAF/MEK/ERK MAP kinases pathway plays a central role in the biology of various cell types, including regulation of the proliferation of melanocytes. Several new inhibitors targeting mutated molecules in the RAS/RAF/MEK/ERK pathway are being tested in the clinic (Table 1; Figure 1).

Full table
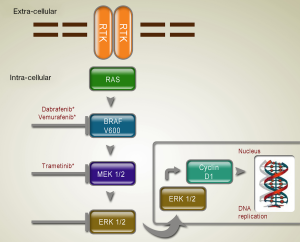
BRAF inhibitor
The selective and potent inhibitor of oncogenic mutant BRAF, vemurafenib and dabrafenib, showed impressive efficacy in clinical studies. A phase III trial, the 2-arm randomized BRAF inhibitor in melanoma-3 (BRIM-3) study, compared the efficacy of vemurafenib therapy to dacarbazine chemotherapy as first-line therapy in metastatic melanoma patients. A total of 675 patients with unresectable, previously untreated stage IIIC or stage IV metastatic melanoma bearing BRAF V600E mutation were enrolled in the study. In the vemurafenib group, most patients had a detectable decrease in tumor size, and 48% of patients experienced an objective response. Both median PFS (5.3 months in vemurafenib group vs. 1.6 months in dacarbazine group) and OS (84% vs. 64%) were improved in vemurafenib group, as compared with the dacarbazine group (15).
Dabrafenib is another inhibitor against BRAF. It is an ATP-competitive inhibitor of BRAF, developed on the basis of its selectivity for inhibiting mutant BRAF in kinase screening panels, cell lines and xenografts. Dabrafenib is known to inhibit V600E, V600K and V600D mutants of BRAF in vitro (18). In a phase 3 randomized controlled trial comparing dabrafenib with dacarbazine, 250 patients with unresectable previously untreated melanoma bearing BRAF V600E mutation were randomized at a 3:1 ratio to receive either oral dabrafenib or dacarbazine. Median PFS was 5.1 months for dabrafenib group and 2.7 months for dacarbazine group, with a hazard ratio (HR) of 0.30 (P<0.001). Although vemurafenib and dabrafenib seem to have similar efficacy in terms of ORR in the two phase 3 trials, the rates of cutaneous squamous cell carcinaoma (cuSCC) /keratoacanthoma, an important adverse effect of vemurafenib, were different (18-25% in vemurafenib trials vs. 6-11% in dabrafenib trials) (16).
MEK inhibitor
In preclinical mouse models, mutations of BRAF were associated with enhanced sensitivity to MEK inhibition; pharmacological MEK blockade was found to completely abrogate tumor growth in BRAF mutant exenografts. Trametinib is an orally available, small-molecule, selective inhibitor of MEK1 and MEK2, and has received FDA approval on May 29, 2013 as the first-line treatment of patients with unresectable or metastatic melanoma harboring a BRAF V600E/K mutation (17). Currently, trametinib is described for patients who have received but failed to previous BRAF inhibitor therapy. Efficacy of combination therapy with dabrafenib and trametinib has been investigated, suggesting that the combination can delay the development of resistance to BRAF kinase inhibition treatment and reduce dose-limiting toxic effect of trametinib as cuSCCs (19).
Until now, there is no study demonstrating the definite effect of BRAF or MEK inhibitor in mucosal melanoma, which may be due to the lower mutation frequency in mitogen-activated protein kinase (MAPK) pathway in mucosal melanoma. Mucosal melanoma would be less likely to respond to therapeutic interventions that target upstream components of the MAPK pathway.
KIT targeted therapies
KIT mutations are frequent in mucosal melanoma subtype but are rarely reported in cutaneous melanoma. Mutations of C-KIT in melanoma are variable, and only selected KIT alterations are practically oncogenic and serve as an effective therapeutic target. The understanding of genetic profile of mucosal melanoma has led to the development of promising molecular agents targeting overactive mutated C-KIT. Imatinib mesylate is a competitive C-KIT inhibitor. Several studies have implicated the efficiency of imatinib in mucosal melanoma. In the phase II trial conducted by Carvajal et al., 28 patients with mutations in or amplification of C-KIT, who had advanced unresectable melanoma arising from acral (10 of 28), mucosal (13 of 28) and chronically sun-damaged sites (5 of 28), were treated with imatinib (400 mg twice daily). Two patients achieved durable complete responses, two achieved durable partial responses, two achieved transient partial responses, and five achieved stable disease lasting for 12 or more weeks. The overall durable RR was 16%, with a median time to progression of 12 weeks, and a median OS of 46.3 weeks (20). In the phase II trial conducted by Peking University Cancer Hospital, 43 patients with metastatic melanoma harboring C-KIT mutations or amplifications were enrolled, including 11 cases of mucosal melanoma. The median PFS was 3.5 months, and the 6-month PFS rate was 36.6%. Rate of total disease control was 53.5%: 10 patients (23.3%) achieved partial response, 13 patients (30.2%) achieved stable disease, and 20 patients (46.5%) had progressive disease (21). These trials indicated for clinical benefits of imatinib in mucosal melanoma.
PI3K-AKT pathway targeted therapies
The PI3K-AKT pathway is one of the most important signaling networks in mucosal melanoma. Few clinical trials in melanoma patients have investigated the activity of agents that act primarily or directly through inhibition of PI3K-AKT activity.
AKT inhibitor
MK-2206 is an oral, highly selective inhibitor of AKT that binds at a site in the pleckstrin-homology (PH) domain, distinct from the ATP-binding pocket, resulting in a conformational change that prevents the localization of AKT to the plasma membrane and its subsequent activation (22). A phase I multicenter trial of 72 patients with advanced solid tumors (including six melanomas) demonstrated that MK-2206 in combination with standard cytotoxic chemotherapies can be safely administered to patients with advanced solid tumors at doses demonstrating antitumor activity (23).
mTOR inhibitor
RAD001 (everolimus) is an mTOR inhibitor that has received significant attention as a potential drug for melanoma. A phase II multicenter trial of 24 patients with metastatic melanoma has been undertaken, and the planned interim analysis after 20 patients were enrolled showed no objective clinical responses, though there was significant disease stabilization (24). The activation of PI3K-AKT pathway in the 24 patients was not evaluated. A patient with nasal cavity primary mucosal melanoma bearing C-KIT L576P mutation, with metastases in mediastinal lymph nodes and lung, received a targeted treatment of imatinib. The patient had a best response of stable disease (aggregate tumor shrinkage of 21%), maintained for 8.5 months. Increased phosphorylation of S6RP, 4E-BP1, Akt and ERK1/2 by immunohistochemistry was displayed in FFPE (formalin fixed paraffin embedded) nasal tissue samples obtained after imatinib treatment (imatinib resistant), as compared with tissue samples obtained before imatinib treatment (imatinib responsive). The patient received the mTOR inhibitor everolimus constituted a partial response (25). Selection of patients with mucosal melanoma harboring activation in the mTOR pathway may improve the efficiency of everolimus.
Conclusion 2: KIT inhibitor showed efficiency in metastatic mucosal melanoma, and mTOR inhibitor may be a potential therapy in mucosal melanoma harboring activation in the mTOR pathway.
Immunotherapies
Melanoma evolves to exploit multiple mechanisms to avoid immune cell recognition and antitumor effector functions, thereby limiting the clinical benefits of immunotherapy strategies. Though a number of immunotherapeutic strategies have been shown to increase the immune system’s ability to control melanoma, immunomodulatory antibodies that directly enhance the function of T cells have been garnering significant attention. These agents are commonly called “checkpoint inhibitors” because they block normally negative regulators of T cell immunity such as cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1) (Figure 2).
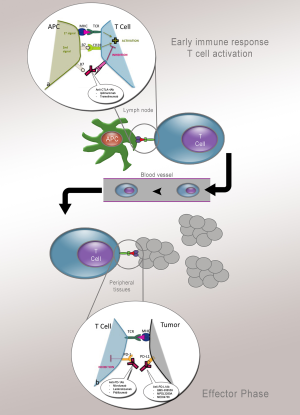
Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4)
CTLA-4 is a member of the CD28:B7 immunoglobulin superfamily and is normally expressed at low levels of the surface of naïve effector T cells and regulatory T cells (Tregs). After stimulation of a naïve T cell through the T cell receptor (TCR), CTLA-4 localizes to the plasma membrane and competes with CD28 for B7, ultimately turning off T cell receptor signaling (26). Antibodies that target CTLA-4 prevent the attenuating function of CTLA-44 and consequently enhance T cell function. CTLA-4 thereby serves as a physiologic “brake” on the activated immune system to maintain normal immune homeostasis (27).
Two antibodies that block CTLA-4, ipilimumab and tremelimumab, have been evaluated in clinical trials, and ipilimumab has been approved by the United States FDA for the treatment of advanced melanoma. Ipilimumab, a monoclonal antibody (mAb) against CTLA-4, was the first agent approved for the treatment of unresectable or metastatic (advanced) melanoma, and showed an OS benefit in a randomized phase III trial (28). Clinical trial data have consistently shown that ipilimumab confers meaningful clinical benefit to patients with advanced melanoma.
Calvin’s investigation found upregulation of CTLA-4, IL-17A, IL-17C, and IL-17E in mucosal melanoma. It may suggest that mucosal subtype patients may have had low antitumor immunity and might benefit from CTLA-4 blockade (29). A retrospective analysis by Michael et al. of 33 patients with unresectable or metastatic mucosal melanoma treated with ipilimumab reported 1 immune-related complete response, 1 immune-related partial response, 5 immune-related stable diseases and 23 immune-related progressive diseases. The median OS from the time of the first dose of ipilimumab was 6.4 months (30). A recent study of Italy showed ipilimumab may be a feasible treatment option in pretreated patients with metastatic mucosal melanoma. There were 855 patients participating in the study, including 71 (8%) metastatic mucosal melanomas. The RR was 12% (some in mucosal subgroup), and the immune-related disease control rate (DCR) was 36%. Median PFS and OS were 4.3 and 6.4 months, respectively. The survival outcomes had no different between melanoma subtypes (31).
PD-1/PD-L1
Whereas CTLA-4 is operational during early activation of T cells in lymphatic tissues, another regulatory molecule expressed on T cells, PD-1, functions during the effector phase of T-cell activation. The interaction of PD-1 with its two ligands, B7-H1 and B7-DC [programmed cell death-ligand 1 (PD-L1) and PD-L2], occurs predominantly in peripheral tissues including the tumor microenvironment and leads to apoptosis and downregulation of T-cell effector function. PD-L1 upregulation in metastatic melanoma was found to be colocalized with tumor-infiltrating lymphocytes and IFN-γ production, indicating a potential resistance mechanism adopted by the tumor to evade the endogenous immune response (32). The anti-PD-1 mAb (nivolumab and lambrolizumab) and anti-PD-L1 mAb MDX-1105 show efficiency in metastatic melanoma (33-35). But the PD-1/PD-L1 blockade therapy for patients with metastatic mucosal melanoma is not evaluated and warrants further investigation in clinical trials.
Conclusion 3: anti-CTLA-4 confers meaningful clinical benefit to patients with advanced mucosal melanoma while the effects of anti-PD-1/PD-L1 in mucosal melanoma need further investigation.
Angiogenesis targeted therapies
Angiogenesis, the process of formation of neovasculature from pre-existing blood vessels, is widely considered as an essential process to ensure the supply of nutrients and oxygen to rapidly growing tumors as well as to provide a route for tumor cell metastasis. Angiogenesis represents a relevant process to modulate in melanoma, as pro-angiogenic ligands and their receptors are overexpressed and have been found to correlate with disease progression and prognosis. Several clinical trials investigating anti-angiogenic strategies in melanoma have been reported.
Bevacizumab
Bevacizumab is a recombinant humanized mAb to VEGF composed of human IgG1 framework regions and antigen binding complementary determining regions from a murine mAb that blocks the binding of all human VEGF isoforms to their cognate receptors. A growing number of clinical trials in advanced melanoma have incorporated bevacizumab in combination with cytotoxic chemotherapy and other targeted agents. Recently, a larger randomized phase 2 study, comparing carboplatin plus paclitaxel chemotherapy with (CPB) or without (CP) bevacizumab as first line treatment for metastatic melanoma in 240 patients, showed improved PFS, OS and ORRs in CPB (36). In a phase 3 clinical trial of bevacizumab combined with a cremaphor-free nanoparticle albumin-bound paclitaxel as first-line therapy in patients with metastatic melanoma, over half of the enrolled patients had extremely poor prognosis disease while the 12-month survival rate was about 83% (37), which was extremely encouraging for bevacizumab-based therapy.
Endostatin
Endostatin, a representative of endogenous angiogenesis inhibitors, is the 20 kDa internal fragment of the C-terminus of collagen XVIII (38). A phase II trial evaluated the efficacy of recombinant human endostatin (Endostar) plus dacarbazine in patients with metastatic melanoma that contains no mutations in c-kit and BRAF genes. A total of 110 patients were included, including 16 cases of mucosal melanomas. As compared to dacarbazine, Endostar plus dacarbazine is well tolerated in patients with metastatic melanoma and yields a significant improvement in PFS and OS (39).
Conclusion 4: There are not sufficient clinical studies to determine benefits of anti-angiogenic therapies in metastatic mucosal melanoma, and larger phase 3 randomized trials are required to evaluate angiogenesis-targeted therapy.
Conclusions
In summary, chemotherapies have similar effects in cutaneous melanoma and mucosal melanoma, but failed to significantly improve outcomes. Targeted therapy of KIT inhibitor showed efficiency in metastatic mucosal melanoma, and mTOR inhibitor may be a potential therapy in mucosal melanoma harboring activation in the mTOR pathway. Immunotherapy of checkpoint inhibitors and anti-angiogenic therapies in metastatic mucosal melanoma need further investigation. So, we can make a primary algorithm of metastatic mucosal melanoma treatments (Figure 3).
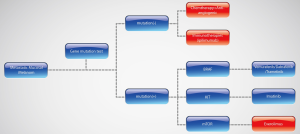
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- DeMatos P, Tyler DS, Seigler HF. Malignant melanoma of the mucous membranes: a review of 119 cases. Ann Surg Oncol 1998;5:733-42. [PubMed]
- Chi Z, Li S, Sheng X, et al. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. BMC Cancer 2011;11:85. [PubMed]
- Yang AS, Chapman PB. The history and future of chemotherapy for melanoma. Hematol Oncol Clin North Am 2009;23:583-97. [PubMed]
- Del Prete SA, Maurer LH, O’Donnell J, et al. Combination chemotherapy with cisplatin, carmustine, dacarbazine, and tamoxifen in metastatic melanoma. Cancer Treat Rep 1984;68:1403-5. [PubMed]
- Lattanzi SC, Tosteson T, Chertoff J, et al. Dacarbazine, cisplatin and carmustine, with or without tamoxifen, for metastatic melanoma: 5-year follow-up. Melanoma Res 1995;5:365-9. [PubMed]
- Saba HI, Cruse CW, Wells KE, et al. Treatment of stage IV malignant melanoma with dacarbazine, carmustine, cisplatin, and tamoxifen regimens: a University of South Florida and H. Lee Moffitt Melanoma Center Study. Ann Plast Surg 1992;28:65-9. [PubMed]
- Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 1999;17:2745-51. [PubMed]
- Bajetta E, Di Leo A, Zampino MG, et al. Multicenter randomized trial of dacarbazine alone or in combination with two different doses and schedules of interferon alfa-2a in the treatment of advanced melanoma. J Clin Oncol 1994;12:806-11. [PubMed]
- Buzaid AC, Legha S, Winn R, et al. Cisplatin, vinblastine and dacarbazine alone in metastatic melanoma: Preliminary results of a phase III Cancer Community Oncology Program (CCOP) trial. Proc Am Soc Clin Oncol 1993;12:389.
- Thomson DB, Adena M, McLeod GR, et al. Interferon-alpha 2a does not improve response or survival when combined with dacarbazine in metastatic malignant melanoma: results of a multi-institutional Australian randomized trial. Melanoma Res 1993;3:133-8. [PubMed]
- Young AM, Marsden J, Goodman A, et al. Prospective randomized comparison of dacarbazine (DTIC) versus DTIC plus interferon-alpha (IFN-alpha) in metastatic melanoma. Clin Oncol (R Coll Radiol) 2001;13:458-65. [PubMed]
- Yi JH, Yi SY, Lee HR, et al. Dacarbazine-based chemotherapy as first-line treatment in noncutaneous metastatic melanoma: multicenter, retrospective analysis in Asia. Melanoma Res 2011;21:223-7. [PubMed]
- Chang W, Lee SJ, Park S, et al. Effect of paclitaxel/carboplatin salvage chemotherapy in noncutaneous versus cutaneous metastatic melanoma. Melanoma Res 2013;23:147-51. [PubMed]
- Homsi J, Bedikian AY, Kim KB, et al. Phase 2 open-label study of weekly docosahexaenoic acid-paclitaxel in cutaneous and mucosal metastatic melanoma patients. Melanoma Res 2009;19:238-42. [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107-14. [PubMed]
- King AJ, Arnone MR, Bleam MR, et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS One 2013;8:e67583. [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [PubMed]
- Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011;305:2327-34. [PubMed]
- Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 2011;29:2904-9. [PubMed]
- Okuzumi T, Fiedler D, Zhang C, et al. Inhibitor hijacking of Akt activation. Nat Chem Biol 2009;5:484-93. [PubMed]
- Molife LR, Yan L, Vitfell-Rasmussen J, et al. Phase 1 trial of the oral AKT inhibitor MK-2206 plus carboplatin/paclitaxel, docetaxel, or erlotinib in patients with advanced solid tumors. J Hematol Oncol 2014;7:1. [PubMed]
- Markovic SN, Suman VJ, Ingle JN, et al. Peptide vaccination of patients with metastatic melanoma: improved clinical outcome in patients demonstrating effective immunization. Am J Clin Oncol 2006;29:352-60. [PubMed]
- Si L, Xu X, Kong Y, et al. Major response to everolimus in melanoma with acquired imatinib resistance. J Clin Oncol 2012;30:e37-40. [PubMed]
- Linsley PS, Bradshaw J, Greene J, et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 1996;4:535-43. [PubMed]
- Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol 1999;162:5813-20. [PubMed]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Wei C, Sirikanjanapong S, Lieberman S, et al. Primary mucosal melanoma arising from the eustachian tube with CTLA-4, IL-17A, IL-17C, and IL-17E upregulation. Ear Nose Throat J 2013;92:36-40. [PubMed]
- Postow MA, Luke JJ, Bluth MJ, et al. Ipilimumab for patients with advanced mucosal melanoma. Oncologist 2013;18:726-32. [PubMed]
- Del Vecchio M, Di Guardo L, Ascierto PA, et al. Efficacy and safety of ipilimumab 3mg/kg in patients with pretreated, metastatic, mucosal melanoma. Eur J Cancer 2014;50:121-7. [PubMed]
- Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [PubMed]
- Kim KB, Sosman JA, Fruehauf JP, et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol 2012;30:34-41. [PubMed]
- Boasberg P, Cruickshank S, Hamid O, et al. Nab-paclitaxel and bevacizumab as first-line therapy in patients with unresectable stage III and IV melanoma. J Clin Oncol 2009;27:abstr 9061.
- O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997;88:277-85. [PubMed]
- Cui C, Mao L, Chi Z, et al. A phase II, randomized, double-blind, placebo-controlled multicenter trial of Endostar in patients with metastatic melanoma. Mol Ther 2013;21:1456-63. [PubMed]


