Analysis of cardiac adverse events following postmastectomy hypofractionated radiotherapy
Introduction
Post-operative radiotherapy (RT) to chest wall after modified radical mastectomy is usually given in 5 weeks with a fraction size of 2 Gy delivered in 25 fractions with or without a boost. This conventionally fractionated RT though proven effective, causes inconvenience to patients (1) of undergoing daily treatment for 5 to 7 weeks, and moreover add-ons to health care expenditures (2). Based on the results of various randomized trials, use of hypofractionated RT has gained significant popularity as adjuvant treatment following breast conserving surgery (3-6). But the data on how hypofractionated RT used post-mastectomy affects the normal tissues is still limited. Majority of the trials are retrospective in nature (7).
Linear Quadratic Model is often used to formulate the equivalent regimes based on the α/β values of early and late reacting tissues. α/β ratio is nothing but the dose D at which the components of cell killing that are proportional to dose i.e., αD equals to those that are proportional to square of dose i.e., βD2. Healthy tissues of breast and rib cage are sensitive to fraction size with α/β values 5 Gy or less (8). Thus, small changes in fraction sizes can produce relatively large changes in effects of RT on these tissues. Chest wall irradiation post-mastectomy exposes normal tissues to doses which can lead to acute as well as long term complications. Opposed tangential portals exposes organ system like heart to significant amount of radiation doses especially when left sided chest wall is treated, raising the normal tissue complication probability. Though new irradiation modalities like three dimensional conformal RT intensity modulated RT have come a long way in patient management, providing better normal tissue sparing with equivalent results, use of two dimensional treatment planning with cobalt 60 is still prevalent in developing countries like India, where procurement of costly machines especially in government setup is still a oncologist’s dream. Cardiac complications post RT are inevitable, with majority of patients being underdiagnosed. Given the long favorable overall survival post treatment in breast cancer patients, the impact on quality of life of patients due to cardiac complications should be reported and quantified.
In its tolerance to radiation, heart is intermediate between kidney and central nervous system. The α/β ratio for heart is low <2 Gy. The threshold dose may be as low as 20 Gy if more than 50% of heart is irradiated, but higher for partial exposure. Radiation induced cardiomyopathy results from dense and diffuse fibrosis, a slowly evolving lesion leading to impaired function.
Use of hypofractionated RT with conventional planning techniques can have an impact on normal tissue toxicity. And to evaluate and quantify the burden of hypofractionation on cardiac tissues is the main aim of this prospective study, utilizing echocardiography (ECHO) which is a useful, non-invasive and repeatable method to identify and monitor left ventricular systolic and diastolic dysfunctions.
Materials and methods
Patients
Following mastectomy 61 female patients with breast cancer, were enrolled from June 2011 to June 2012, after having obtained informed consent. The study was approved by local research ethical board. Eligibility criteria included- post-mastectomy female patients, >20 years age, having either stage 1, 2 or 3, histologically proven disease. Patients having positive margins, chronic heart disease, history of previous breast cancer, pregnant lactating, those with chest malformations, and patients who had undergone breast conserving surgery were excluded from this study. All patients received and completed chemotherapy before starting radiation.
All patients had ECHO before the start of RT, then at three monthly intervals to assess baseline status and early and late adverse effects. The median follow up was 20 months. The Common Terminology Criteria for Adverse Events 3.0 was employed to evaluate early and late effects of RT on left ventricular ejection fraction (LVEF). Percentage decline in ejection fraction (EF) was used to grade cardiac adverse events. Grade (Gr) 1 included resting EF <60-50%, Gr 2 EF <50-40%, Gr 3 EF <40-20% and Gr 4 EF <20%.
Radiotherapy (RT)
All patients underwent hypofractionated RT post-mastectomy either 40 Gy in 15 fractions (dose per fraction 2.65 Gy, total time 22 days), or 42.5 Gy in 16 fractions (dose per fraction 2.66 Gy, total time 19 days), depending on treating physician’s preference. External beam RT was administered by Teletherapy Theratron 780e or Equinox Cobalt60 machines. Intention was to treat the chest wall post-mastectomy along with the regional lymph nodes, as per the indications. Separate fields were placed to irradiate the chest wall and supraclavicular/axillary area. Patients were placed on wedged breast board with hand above head. Radiation fields were drawn on the skin and contours were taken. Tangential RT portals were used for chest wall irradiation and direct portal for supraclavicular/axillary fields. Bolus was used on alternate days. Two dimensional planning was done.
Echocardiography (ECHO)
All patients underwent ECHO as per protocol. Left ventricular systolic function resting, as well as percentage drop of values from baseline was measured, percentage computed when the amount of blood ejected during a ventricular contraction of the heart is compared to the amount that was present prior to the contraction. EF is the fraction of the end-diastolic volume (EDV) that is ejected with each beat; that is, it is stroke volume (SV) divided by EDV.
Statistical analysis
Descriptive statistics were used to present the data. For repeated measurements ANOVA-analysis of variance was utilized so as to assess the difference in EF values at different times, along with to correlate LVEF values at different times with age, smoking history, hypertension, diabetes history, body mass index, chemotherapy and hormonal therapy and with laterality of disease. Pearson chi-square test was used to assess correlation of numerical variables.
Results
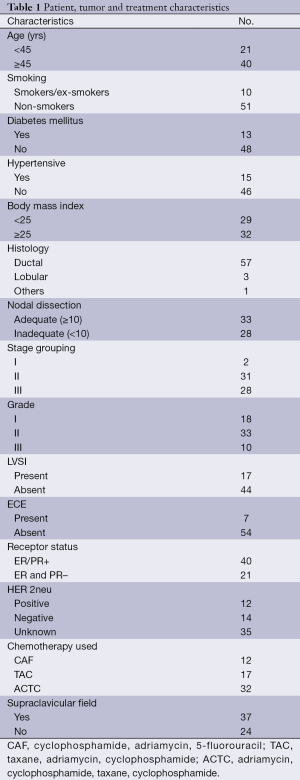
A total of 61 patients were screened in our present study. Patient, tumor, and treatment characteristics are reported in Table 1. Majority of patients were in 45 to 60 years age group (68%). With a median age of 49 years old, median follow up was 20 months. Average length and width of tangential field were 19.5 cm ± 1.5 cm, and 6.8 cm ± 1.2 cm respectively. Majority of the patients were in stage 2 (50.81%). Main histology was ductal 93.44%. A total of 33 patients had adequate lymph node dissection defined as ≥10 lymph nodes dissected. A total of 54.09% had Gr 2 disease, with 17 patients having lympho-vascular invasion, and 7 with extracapsular extension. Supraclavicular field was utilized in 60.7% of patients. Majority of the patients were treated with taxanes and all chemotherapy was completed before starting radiation.
Full table
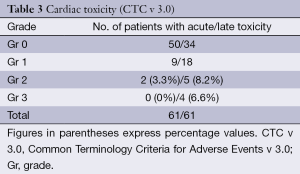
The mean of LVEF values before and after RT are summarized in Table 2. There was no statistically significant difference in the mean values even at two years of follow up (all measurements being expressed as percentage values). The CTC (v 3.0) cardiac adverse effects, acute and chronic grading is depicted in Table 3. A total of 3.3% developed Gr 2 acute adverse events while 14.75% had ≥ Gr 2 late adverse events. One patient developed skin metastasis 3 months after completion of treatment and later had pericardial effusion. However, symptomatic adverse cardiac events were very rare in our study.

Full table
Full table
Discussion
There has always been a deep concern about cardiotoxicity post RT in breast cancer patients. Due to contour of the chest wall some portion of the heart has to be included in the tangential portals. Cardiac irradiation can result in significant pathologic damage to the heart as manifested by microcirculatory damage leading to ischemia, fibrosis, diffuse myocardial interstitial fibrosis, pericarditis, pericardial effusion, fibrous thickening of pericardium, valvular fibrosis, and accelerated atherosclerosis. An increased risk of ischemic heart disease has been demonstrated in EBCTCG’s meta-analysis (9) amongst women receiving adjuvant radiation to the breast. Meta-analysis by Haybittle et al. (10) revealed cases of fatal myocardial infarction associated with adjuvant RT to the breast. In the Oslo and Stockholm trials, the retrospective analysis showed that increase in non-breast cancer deaths was due to cardiac mortality. Gustavsson et al. (11) reported no cardiac deaths in patients treated postoperatively with adjuvant breast radiation.
Hypofractionation with fraction sizes >2 Gy was introduced to lessen the burden of treatment, for convenience of patients, and moreover to have efficient use of resources. Majority of trials have provided data on cardiac toxicity for conventionally fractionated RT, and in patients who underwent breast conserving surgery. Though the two large trials start A and B included post-mastectomy patients (15% and 8% respectively), no separate toxicity analysis was done by the authors in this patient population. Data pertaining to adverse cardiac effects of hypofractionated RT post-mastectomy is scarce. Tjessem et al. (7) provided the results of long term mortality from ischemic heart disease after hypofractionated radiation therapy in breast cancer and concluded that degree of fractionation and photon beams contributed to increased ischemic heart disease mortality but the results were retrospective in nature. Different techniques have been used in different trials to assess the cardiotoxicity of RT. In our present study cardiac status was assessed employing ECHO performed by a trained operator as a standard procedure for evaluating cardiotoxicity following hypofractionated RT.
A total of 40 Gy/15 fractions or 42.5 Gy/16 fractions were utilized in adjuvant settings post-mastectomy. While modeling the schedules α/β values of 3 Gy for late changes and 10 Gy for early changes were considered. Using these values the biologically effective doses estimated are as follows; early/late effects 53.8 Gy/80.3 Gy and 50.6 Gy/75.3 Gy for 40 and 42.5 Gy schedules respectively.
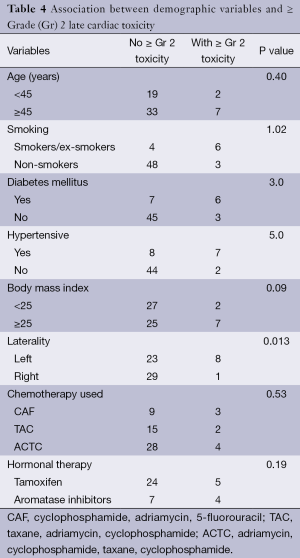
In our present study percentage decline in values of LVEF from baseline were investigated to quantify the hypofractionated RT induced early and late cardiac toxicity. We observed that the variation in LVEF values presented as mean over a 2 years follow up period and expressed as percentage decline from baseline, was not statistically significant. However, individual assessment and bifurcation of acute and late reactions showed that 3.3% of the study population developed ≥ Gr 2 acute reactions and in patients with left sided disease 6.5% had ≥ Gr 2 acute reactions. Overall 8.2% and 6.6% developed Gr 2 and Gr 3 late adverse events, in contrast to 12.9% Gr 2 and 12.9% Gr 3 respectively, late toxicity in left sided diseased patients. Present study showed statistically significant difference in late cardiac toxicity when comparing left-sided with right-sided disease (P=0.013). This is in agreement with the results published by Darby et al. (12), who demonstrated an increased risk of cardiac death after treatment for left-sided breast cancer. Though with Gr 1 and 2 adverse cardiac events patients are usually asymptomatic, any Gr 3 or above and symptomatic adverse events warrants intervention.
Radiation potentiates the cardiotoxic effects of certain chemotherapeutic agents, such as anthracyclines (13). This interaction appears to be dependent on the total cumulative dose of anthracyclines (14). In our study one of the three chemotherapeutic regimes-CAF (cyclophosphamide, adriamycin, 5-FU), TAC (taxane, adriamycin, cyclophosphamide) or ACTC (adriamycin, cyclophosphamide, taxane, cyclophosphamide) was used depending on disease status or physicians preference. The total cumulative dose of anthracycline did not exceeded 360 mg/m2. Our trial found no statistically significant late adverse effect in patients receiving chemotherapy, with no influence of the type of regime used (P=0.53). As for the impact of hormonal therapy was concerned, no statistically significant difference was found.
When considering other risk factors contributing to radiation induced cardiac adverse events, age at irradiation has been demonstrated to increase the risk. A study by Hooning et al. (15) reported that patients younger than 35 years have a relative risk of 6.5 as compared to general population for radiation induced heart disease. In our series, 2 out of 19 patients aged <45 years old had ≥ Gr 2 late cardiac toxicity which was not statistically significant (P=0.40) (Table 4).
Full table
King et al. (16) in a study published in 1996, showed that risk factors like diabetes mellitus, hypertension, and overweight, influence the overall risk of radiation induced heart disorders. Out of a total 15 patients with a history of hypertension 7 developed ≥ Gr 2 late toxicity, while 6 out of a total of 13 with history of diabetes had ≥ Gr 2 late cardiac events. Late toxicity ≥ Gr 2 was reported in 22% of the total patients with body mass index ≥25, with results approaching clinical significance (P=0.09). Smoking also increases the relative risk. Out of 10 patients who were smokers or ex-smokers, 6 developed ≥ Gr 2 late cardiac toxicity.
Opposed tangential portals used for chest wall irradiation exposes cardiac tissues to significant amount of radiation doses. Cardiac tissue is particularly sensitive to fractionation (α/β <3 Gy), clinically significant incidence of radiation toxicity even after use of hypofractionated RT post-mastectomy was not recorded in our study. Studies from Darby et al. (12) have found that it can take decades to develop cardiac damage after RT. Though the maximum follow up period in our study was only 2 years, more longer follow up data is still needed to firmly establish the safety of hypofractionated RT following mastectomy in breast cancer patients, which is one of the limitations of this present study. The study group is also small, which is again a limitation to firmly conclude the findings. As of now, results from our study clearly support the use of hypofractionated RT post-mastectomy with clinically insignificant adverse cardiac events.
Conclusions
Hypofractionated RT regimes per se are not detrimental to the effective and safe delivery of post-mastectomy chest wall irradiation, especially when concerning acute or late cardiac tissue damage, though a follow up period of 2 years is too short to assess potential late cardiac damage.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Whelan TJ, Levine M, Julian J, et al. The effects of radiation therapy on quality of life of women with breast cancer: results of a randomized trial. Ontario Clinical Oncology Group. Cancer 2000;88:2260-6. [PubMed]
- Suh WW, Pierce LJ, Vicini FA, et al. A cost comparison analysis of partial versus whole-breast irradiation after breast-conserving surgery for early-stage breast cancer. Int J Radiat Oncol Biol Phys 2005;62:790-6. [PubMed]
- Bentzen SM, Agrawal RK, Aird EG, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet Oncol 2008;9:331-41. [PubMed]
- Bentzen SM, Agrawal RK, Aird EG, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet 2008;371:1098-107. [PubMed]
- Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumor control in patients with early-stage breast cancer after local tumor excision: long-term results of a randomised trial. Lancet Oncol 2006;7:467-71. [PubMed]
- Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst 2002;94:1143-50. [PubMed]
- Tjessem KH, Johansen S, Malinen E, et al. Long-term cardiac mortality after hypofractionated radiation therapy in breast cancer. Int J Radiat Oncol Biol Phys 2013;87:337-43. [PubMed]
- Thames HD, Bentzen SM, Turesson I, et al. Time-dose factors in radiotherapy: a review of the human data. Radiother Oncol 1990;19:219-35. [PubMed]
- Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 2000;355:1757-70. [PubMed]
- Haybittle JL, Brinkley D, Houghton J, et al. Postoperative radiotherapy and late mortality: evidence from the Cancer Research Campaign trial for early breast cancer. BMJ 1989;298:1611-4. [PubMed]
- Gustavsson A, Bendahl PO, Cwikiel M, et al. No serious late cardiac effects after adjuvant radiotherapy following mastectomy in premenopausal women with early breast cancer. Int J Radiat Oncol Biol Phys 1999;43:745-54. [PubMed]
- Darby S, McGale P, Peto R, et al. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: Nationwide cohort study of 90 000 Swedish women. BMJ 2003;326:256-7. [PubMed]
- Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited stage Hodgkin’s lymphoma. N Engl J Med 2012;366:399-408. [PubMed]
- Shapiro CL, Hardenbergh PH, Gelman R, et al. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol 1998;16:3493-501. [PubMed]
- Hooning MJ, Botma A, Aleman BM, et al. Long term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007;99:365-75. [PubMed]
- King V, Constine LS, Clark D, et al. Symptomatic coronary artery disease after mantle irradiation for Hodgkin’s disease. Int J Radiat Oncol Biol Phys 1996;36:881-9. [PubMed]


