Functional and molecular imaging in cancer drug development
Introduction
Biomarkers are becoming an indispensable part of drug development. A biomarker is a representative that serves as an indicator of a patho-physiological process, or as a response to treatment which affects such a process (1). A more ideal use of imaging biomarkers for drug development can serve multi-purposes, such as disease staging, patient stratification, risk assessment, pharmacokinetics/pharmacodynamics (PK/PD), drug safety and efficacy. The use of non-invasive imaging biomarkers to assess drug therapies has become more common during the last decades. From December 11, 1992, to July 1, 2010, the U.S. Food and Drug Administration (FDA) granted accelerated approval of 47 new indications for 35 anticancer drugs using surrogate endpoints, and most of them were objective response rate and progression-free survival (PFS), typically measured by magnetic resonance imaging (MRI) or computer tomography (CT) (2). However, vigorous debate has challenged the use of anatomic assessments alone, as it may take two or three months to detect any shrinkage, thus only morphological information can be obtained. But, it may not be a suitable tool to assess response when agents targeting signaling pathways are involved, most notably in patients with gastrointestinal stromal tumor (GIST) treated by cytostatic targeted agents (3). To better understand tumor microenvironment (TME), and thereby select specific agents targeting metabolic key pathways, morphological information is not enough. Therefore, the addition of functional information on TME through imaging biomarkers would aid principle investigators to design personalized treatment planning by using specific targeted drugs (4).
Functional imaging of TME has several advantages: (I) it is a non-invasive procedure; (II) various sites of the tumors can be visualized and quantified simultaneously; (III) functional imaging using biomarkers can generate three dimensional images of the tumor which allows better quantification; (IV) moreover, functional imaging is capable of visualizing heterogeneous metabolic processes, such as glucose metabolism or tumor hypoxia, which are important contributors to tumor resistance and progression.
Molecular imaging using various labelled radioactive tracers such as fluorodeoxyglucose (FDG), fluoromisonidazole (FMISO), fluorothymidine (FLT), and functional imaging using advanced techniques such as dynamic contrast enhancing (DCE)-MRI and diffusion weighted (DW)-MRI, gain an increasing importance in cancer drug development (Figure 1). Quantitative measurements of imaging biomarkers compared to mere visual evaluation allow for more objective evaluation of disease, and more accurate monitoring through time (1). Therefore, the purposes of this review are (I) to summarize the basic principle and qualification of various imaging biomarkers; (II) to investigate key metabolic pathways up-regulated in cancer cells by using imaging biomarkers and to facilitate targeted cancer drug development; and (III) to describe pitfalls and recommendations when imaging biomarkers are implemented in multicenter trials.
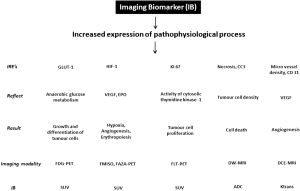
Imaging measurements and qualification
FDG (glucose metabolism)
The most frequently used positron emission tomography (PET) tracer in oncology is FDG for measuring glucose metabolism of the cell (5). However, FDG is not a substrate for metabolism in the glycolytic pathway. Therefore, the degree of trapped FDG uptake in the cells reflects the level of glucose metabolism and could be potentially used as imaging biomarker for early treatment response assessment in cancer patients (6). Maximum standardized uptake value (SUVmax) is a quantitative index to characterize FDG biomarker uptake, hence approximating the glucose metabolism; high SUVmax is associated with aggressive tumor metabolism and poor survival (7,8).
The transport of FDG, a glucose analogue, into cells is mediated by glucose transporters (GLUT-1 and 2) through the plasma membrane (9). Several published studies support significant positive correlation between FDG-PET uptake and the expression of GLUT examined by immunohisto-chemical staining (10-12). Primarily, the overexpression of GLUT characterizes enhanced tumor glucose metabolism and thereby increased FDG uptake is noticed on PET scan.
Demetri et al. (13) showed that, in all GIST patients with a response, the FDG-PET uptake in the tumor had decreased from baseline as early as 24 hours after a single dose of imatinib administration. In addition to that, in all patients, increased FDG-PET uptake from baseline is associated with disease progression. Also, FDG-PET uptake results were correlated with progression on CT or MRI.
Multiple studies have evaluated the role of FDG-PET and showed it promising in assessing response to treatment in solid tumors (14-16). However, the interpretation of SUV is not straightforward, with many factors affecting the values that can be derived. It was shown that a reliable drop in SUV, indicating a tumor response, is only seen in patients with high initial SUV (17). Caution should, therefore, be exercised when we interpret quantitative molecular imaging.
FAZA, FMISO (tumor hypoxia)
Tumor hypoxia is an important adverse prognostic factor and contributes to resistance for both chemotherapy and radiotherapy in several tumor types (18). Under hypoxic cell conditions, tumor hypoxia biomarkers undergo definite reductive metabolic pathways, resulting in reactive tumor metabolite markers which selectively bind to macromolecular cell components that can be detected by the PET signal, but which are washed out from normoxic cells (19,20).
FMISO was the first tracer tested clinically for tumor hypoxia, and it is still widely used (21-23). The novel hypoxia specific tracer, fluoroazomycin arabinoside (FAZA), has generated higher tumor-to-background ratios compared to FMISO in preclinical studies (24,25). FAZA also becomes a more attractive tracer for clinical use due to its more rapid clearance of unbound tracer from non-hypoxic tissues (24).
A clinically relevant exogenous hypoxic biomarker is pimonidazole. With this biomarker, high resolution image of hypoxia distribution at micro-regional level can be obtained using immunohistochemistry. The tumor hypoxia determined by pimonidazole binding assay is consistent with radiobiologically relevant hypoxic volume (26). Dubois et al. (27) found significant correlation between the hypoxic area derived from pimonidazole stained tumor section with the FMISO-PET defined hypoxic volume in an experimental rat tumor model (r=0.9066; P<0.0001).
FLT (tumor cell proliferation)
FLT was introduced by Shields et al. (28) as a PET proliferation imaging biomarker. FLT is monophosphorylated by thymidine kinase 1 (TK1), which leads to intracellular trapping. Since the concentration of TK1 is upregulated during the S phase of the cell cycle, the uptake of FLT reflects proliferation.
Tsuyoshi et al. (29) evaluated the effect of gemcitabine-based secondary chemotherapy with FLT- and FDG-PET imaging biomarkers in patients with stage IIIc recurrent ovarian cancer. FLT SUVmax decreased earlier than FDG SUVmax. Interestingly, FLT SUVmax correlated better with a reduction in size as measured by CT. Given the good imaging properties and strong correlation between functional imaging parameter (proliferation) FLT uptake and CT morphological parameters, SUVmax of FLT appears to be a promising biomarker for monitoring response to gemcitabine-based secondary chemotherapy treatment in recurrent ovarian cancer patients.
The rationale behind the FLT-PET uptake in tumors is based on TK1 activity and Ki-67 index dependence on proliferation. Since the concentration of TK1 and Ki-67 is overexpressed during the active proliferation phase of the cell cycle (S phase), the uptake of FLT is supposed to depend on TK1 and Ki-67 concentration. In a preclinical study, Rasey et al. (30) showed strong correlation between FLT and cell growth, TK1 activity and also with the percentage of cells in S phase of cell cycle (28,31). Recently, Yamamoto et al. (31) demonstrated a significant positive correlation between the proliferation index derived from Ki-67 immunohistochemistry with the FLT-PET uptake (r=0.81, P<0.01) in patients with newly diagnosed and recurrent gliomas (n=56). Given the strong correlation between the FLT uptake and TK1 and Ki-67, FLT appears to be a promising tracer for imaging proliferation.
DW-MRI (cell density)
DW-MRI is an advanced MR technique widely used for the detection and characterization of cancer as well as for monitoring the response to therapy. DW-MRI depends on the microscopic mobility of water in tissues, and it provides a unique imaging biomarker of water interaction with cellular, subcellular and macromolecular entities that impede free water movement (32). In oncologic imaging, DW-MRI has been used to evaluate tumor microstructure, e.g., cell membrane integrity and cellularity, which reflects lesion aggressiveness and tumor response. The acquisition of DW-MRI is non-invasive, does not require any exogenous contrast agents, does not use ionizing radiation, can be obtained relatively rapidly, and is easily incorporated into routine patient evaluation. The apparent diffusion coefficient (ADC) is the quantitative parameter of DW-MRI, and has been shown to be of high potential value for assessing treatment response (33,34). A low ADC reflects restricted diffusion and can be found in hypercellular tissues such as tumors, lymph nodes or in areas of fibrosis. A high ADC reflects less restriction of extracellular water motion and can be found in tissues with high glandular components or distinct necrosis. Cell kill due to efficient drug treatment leads to a loss of cell membrane integrity and reduction in tumor cell density with increase in the interstitial space, and hence it changes ADC measurement in the tumor tissue.
Foroutan et al. (34) evaluated the correlation between ADC and cell death in an osteosarcoma xenotransplant model at pre-treatment and at early time points following treatment. Pixel-by-pixel histograms were produced for each mouse prior to and following the treatment to quantify ADC. Cleaved caspase 3 (CC3) was used as an immunohistochemical marker to quantify cell death. Statistically significant differences in ADC maps were observed between control mice and treated mice, which demonstrates an increase in ADCs towards higher values in treated animals compared to controls. CC3 activity was also significantly higher in the treated animals compared to controls. Overall, a positive correlation was observed between increase in ADC values and cell death depicted by CC3 staining.
DCE imaging (blood flow and vascular permeability)
DCE imaging (MRI, CT and ultrasound) allows non-invasive quantification of TME and its vascular structure and function. The degree of DCE signal intensity reflects the pathophysiological factors, which include tissue perfusion and capillary permeability (35). Serial images are acquired dynamically before, during and after administration of a contrast agent: gadolinium for MRI, iodinated contrast for CT and microbubbles for ultrasound. The acquired data are fitted to mathematical models to obtain quantitative parameters through regions of interest. The volume transfer constant (Ktrans) is often used as a marker for the permeability of tumor vasculature. Other measures used are the rate constant Kep and the initial area under the gadolinium concentration curve (IAUGC).
Understanding the dynamics of tissue parameters is crucial for developing anti-angiogenic drugs. Vascular targeting agents such as bevacizumab or vandetanib are developed to reduce vascular permeability and promote tumor necrosis. Kummar et al. (35) investigated the effect of the anti-angiogenic drug vandetanib in patients with lymphomas. They observed a positive correlation between DCE-MRI parameters and plasma vascular endothelial growth factor (VEGF) levels. Similar results were reported by Donaldson et al. (36) who showed that tumors with poor permeability significantly correlated with the expression of plasma VEGF and the hypoxia marker pimonidazole. High expression of VEGF is associated with tumor angiogenesis and hypoxia, and thereby promotes tumor growth.
Imaging in cancer drug development
Stratifying patients
Molecular and functional imaging provides additional information on tumor characterization, which could help to “pre-select” and “enrich” a patient population. For example, in patients treated with gefitinib, a low baseline SUV of 18F-FDG has been shown to have prognostic value and to be associated with a higher response rate and a prolonged PFS (37).
Identification of tumor hypoxia could facilitate the use of hypoxia stimulated pro-drugs, which selectively kill hypoxic cells. Tirapazamine (TPZ) is such an example. The relatively limited benefit obtained in a trial reported by the CATAPULT I study group was likely due to poor patient stratification with inclusion of patients with better-oxygenated tumors (38). Recently, Rischin et al. (39) compared the cisplatin/5-FU vs. cisplatin/TPZ regimen in patients with head and neck squamous-cell cancer, in which FMISO-PET hypoxic imaging was used to stratify the tumors into hypoxic and non-hypoxic ones. The authors have shown that TPZ improved local tumor control in hypoxic but not in non-hypoxic tumors.
Imaging-guided therapy could promote personalizing treatment, for example by adjusting the treatment for non-responders at an initial phase of treatment. Within the drug development, this sort of response monitoring could be used for selecting a homogeneous patient group for further studies by choosing only those patients who show early metabolic response. Several trials are currently investigating the use of FDG-PET/CT for early response-adapted therapy in lymphoma, with therapeutic stratification based on interim FDG-PET/CT results (40-42). The PET-response-guided treatment has also been investigated in adenocarcinoma of the oesophagogastric junction, and the MUNICON phase II trial showed the feasibility of imaging-guided stratification by using the early metabolic response assessment from FDG-PET to clinical decision making in the treatment of solid tumors (40).
Verifying biological target engagement
The downstream effects of vascular endothelial growth factor receptor (VEGFR) inhibition on DCE-MRI have been documented in more than 30 phase I and II trials with a significant reduction in Ktrans and/or IAUGC being reported with multiple agents (43).
The more direct approach of using PET in cancer drug development is by labelling the drug itself. The anti-human epidermal growth factor receptor 2 (HER2) monoclonal antibody trastuzumab was used to treat breast cancer patients with HER2 expressing tumors and showed improved survival (44). Radionuclide labelled trastuzumab can visualize the affinity of the targeted agent in vivo, which allows us to collect vital information about the pharmacokinetic properties of the drug such as injected dose versus accumulated drug concentration in the organs and its regional bio-distribution. In this case, the use of radionuclide imaging may overcome problems associated with biopsies, including sampling errors and discordance of expression between primary tumors and metastases. Moreover, the drug uptake by the target tissue can be quantified at sequential imaging scans, and it might give us insight into drug’s action at the target tissue and its association with the tumor response.
Defining dose setting
In phase I trials, dose-escalation is usually undertaken to define the maximum tolerated dose (MTD), under the assumption that the most pronounced changes are likely to be detected at the highest dose. But, target saturation may already be reached at lower dose levels. Through direct visualization of target inhibition, imaging changes are likely to be apparent at lower doses than the MTD, and imaging may be used in choosing the optimal biological dose. In a study of brivanib, a dual VEGFR and fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor, Jonker et al. (45) evaluated DCE-MRI responses in several dose schedules in selected patients, known to respond to anti-VEGFR therapies, and then selected the optimal schedule for a phase II trial. Despite this experience, imaging is not commonly used for selecting dose or schedule, and such data are limited, so the use of imaging to determine the optimal schedule of a targeted agent or to monitor drug activity has to be further explored for cancer drug development.
Novel surrogate endpoint for early evaluation of drug activity
A growing understanding of the underlying molecular pathways active in cancer has led to the development of novel therapies targeting VEGFR, EGFR, phosphoinositide 3-kinase (PI3K), mammalian target of rapamycin (mTOR), protein kinase B (Akt) and other pathways. Unlike the cytotoxic chemotherapy, many of these molecular targeted agents are cytostatic, causing inhibition of tumor growth rather than tumor regression. In this context, using tumor shrinkage as a surrogate endpoint may not be the most adequate mean to measure therapeutic response, as the response rates only based on change of tumor size are low, despite a high percentage of patients having prolonged stable disease and sometimes even improvements in survival. Therefore, functional imaging provides a unique potential opportunity to assess antitumoral activity at early stage.
Many have stimulated the FDA to accept novel surrogate endpoints, such as novel imaging endpoints that can be measured earlier than tumor shrinkage and are likely to predict clinical benefit. A qualified biomarker accepted by the FDA as a surrogate endpoint needs to match several important criteria: (I) the endpoint must have an accepted, standardized definition; (II) data from multiple clinical studies must demonstrate a strong correlation of the surrogate endpoint with clinical outcome; (III) well-powered prospective studies must have been performed to validate the surrogate endpoint (i.e., truly predictive of clinical benefit with meaningful improvement in patient outcome) (46). The strength of evidence will vary, depending on whether the surrogate is intended for use in accelerated approval or definite regulatory approval.
FDG uptake (SUV) has been proposed as an appropriate novel surrogate endpoint for early evaluation of drug activity in clinical trials. There have been many retrospective and some prospective studies in a variety of cancer types that have demonstrated a promising correlation between SUV decrease and survival (41,47). To date, these studies have been primarily performed in single institutions with small numbers of patients. To our knowledge, there are two ongoing multicenter trials prospectively designed to validate FDG-PET as a surrogate endpoint in lymphoma (CALGR-53030) and non-small cell lung cancer (RTOG-0235/ACRIN6668). Large prospective multi-center clinical trials are needed to assess the degree of correlation by comparing a pre-defined threshold in SUV change to clinical outcome.
Imaging in multicenter clinical trials
Standardization
Although many imaging biomarkers have been described for cancer research, few of them are widely considered adequate to provide unambiguous assessment of response, and enough for making decisions to stop or continue drug development processes. Implementing molecular and functional imaging to assess response requires that an observed change of the imaging biomarker due to treatments must be greater than the intrinsic and extrinsic variability of the biomarker in the absence of treatment. High reproducibility of molecular and functional imaging techniques relies on good quality data and standardized procedures. Standardization is the first and crucial step when imaging is implemented in multicenter trials. In this context, the EORTC-PET study group issued recommendations for the measurement of [18F]FDG uptake in monitoring treatment response in 1999 (48). These recommendations included suggestions for patient preparation, pre-therapy and post-therapy imaging delays, and techniques for measuring SUV. Following that, guidelines of the National Cancer Institute (NCI) and the European Association of Nuclear Medicine (EANM) for tumor PET imaging enriched the standardized procedures (49,50), making it more feasible to include PET in large multicenter trials. Regarding advanced MRI techniques, such as DCE, the techniques are relatively simple but require strict protocols, careful acquisition, accurate dosing of contrast agent and suitable selection of injection rate, image timing, and image analysis for quantification. In US, the Quantitative Imaging Biomarker Alliance (QIBA) DCE-MRI technical committee provided guidelines and defined basic standards for DCE-MRI measurement and quality control that enable consistent, reliable and fit-for-purpose quantitative measurements when DCE MRI is implemented in multicenter trials (51). In Europe, the Quantitative Imaging in Oncology: Connecting Cellular Processes to Therapy (QuIC-ConCePT) consortium was created and resourced by the Innovative Medicines Initiative (IMI), Europe’s largest public-private initiative (4). It aims to qualify three specific imaging biomarkers of tumor cell proliferation, apoptosis, and necrosis, to allow drug developers to demonstrate reliably the modulation of these pathologic processes in tumors of patients in future trials (4). The precompetitive research and public-private partnerships may reduce the duplication, and develop imaging biomarkers in a most robust, consistent and cost-effective way, so as to accelerate drug development.
Recommendation
Providing a benchmark, based on a set of common principles of implementing functional and molecular imaging in multicenter trials, is important to facilitate exchange of data, promote quality, accelerate research and reduce attrition rate for drug developers. In addition to the summary on the utility of imaging biomarkers based on literature review, we provide general recommendations for principal investigators designing and conducting multicenter clinical trials that include functional and moleculare imaging biomarkers (Table 1).
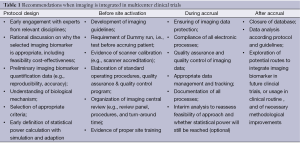
Full table
Conclusions
In the past decade, advances in biology and genomics have led to the development of targeted agents against cancer. This paradigm shift emphasizes the need for specific imaging biomarkers to identify key metabolic changes within the TME and thereby selecting a specific drug of choice. Non-invasive in vivo imaging offers unique, sensitive and clinically transformable information for cancer drug development, notably via efficient patient selection, imaging-guided therapeutic stratification, verification of biological target modulation and dose adaptation. In addition, functional and molecular imaging may potentially allow us to depict accurate changes in tumors, particularly before anatomic changes are evident, and to predict long-term clinical benefit. However, large prospective multicenter studies are needed to further qualify and validate the potential functional imaging biomarkers by demonstrating a strong correlation with clinical outcome. When imaging is implemented in multicenter clinical trials, we highly recommend designing studies with sound methodology and conducting studies with adequate standardization of data acquisition and analysis techniques.
Acknowledgements
The authors like to thank the support of Fonds Cancer (FOCA) from Belgium.
Disclosure: The authors declare no conflict of interest.
References
- Lesko LJ, Atkinson AJ Jr. Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: Criteria, validation, strategies. Annu Rev Pharmacol Toxicol 2001;41:347-66. [PubMed]
- Johnson JR, Ning YM, Farrell A, et al. Accelerated approval of oncology products: The food and drug administration experience. J Natl Cancer Inst 2011;103:636-44. [PubMed]
- Liu Y, Litiere S, de Vries EG, et al. The role of response evaluation criteria in solid tumour in anticancer treatment evaluation: Results of a survey in the oncology community. Eur J Cancer 2014;50:260-6. [PubMed]
- Waterton JC, Pylkkanen L. Qualification of imaging biomarkers for oncology drug development. Eur J Cancer 2012;48:409-15. [PubMed]
- de Geus-Oei LF, van Krieken JH, Aliredjo RP, et al. Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer 2007;55:79-87. [PubMed]
- Bollineni VR, Wiegman EM, Pruim J, et al. Hypoxia imaging using positron emission tomography in non-small cell lung cancer: Implications for radiotherapy. Cancer Treat Rev 2012;38:1027-32. [PubMed]
- Cheng NM, Fang YH, Chang JT, et al. Textural features of pretreatment 18F-FDG PET/CT images: Prognostic significance in patients with advanced T-stage oropharyngeal squamous cell carcinoma. J Nucl Med 2013;54:1703-9. [PubMed]
- Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): A systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008;3:6-12. [PubMed]
- Caracó C, Aloj L, Chen LY, et al. Cellular release of (18F)2-fluoro-2-deoxyglucose as a function of the glucose-6-phosphatase enzyme system. J Biol Chem 2000;275:18489-94. [PubMed]
- Jo MS, Choi OH, Suh DS, et al. Correlation between expression of biological markers and [F]fluorodeoxyglucose uptake in endometrial cancer. Oncol Res Treat 2014;37:30-4. [PubMed]
- Toba H, Kondo K, Sadohara Y, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography and the relationship between fluorodeoxyglucose uptake and the expression of hypoxia-inducible factor-1alpha, glucose transporter-1 and vascular endothelial growth factor in thymic epithelial tumors. Eur J Cardiothorac Surg 2013;44:e105-12. [PubMed]
- Ong LC, Jin Y, Song IC, et al. 2-(18F)-2-deoxy-D-glucose (FDG) uptake in human tumor cells is related to the expression of GLUT-1 and hexokinase II. Acta Radiol 2008;49:1145-53. [PubMed]
- Demetri GD, Heinrich MC, Fletcher JA, et al. Molecular target modulation, imaging, and clinical evaluation of gastrointestinal stromal tumor patients treated with sunitinib malate after imatinib failure. Clin Cancer Res 2009;15:5902-9. [PubMed]
- Abe K, Baba S, Kaneko K, et al. Diagnostic and prognostic values of FDG-PET in patients with non-small cell lung cancer. Clin Imaging 2009;33:90-5. [PubMed]
- Suga K, Kawakami Y, Hiyama A, et al. Dual-time point 18F-FDG PET/CT scan for differentiation between 18F-FDG-avid non-small cell lung cancer and benign lesions. Ann Nucl Med 2009;23:427-35. [PubMed]
- Jager PL, Gietema JA, van der Graaf WT. Imatinib mesylate for the treatment of gastrointestinal stromal tumors: Best monitored with FDG PET. Nucl Med Commun 2004;25:433-8. [PubMed]
- McDermott GM, Welch A, Staff RT, et al. Monitoring primary breast cancer throughout chemotherapy using FDG-PET. Breast Cancer Res Treat 2007;102:75-84. [PubMed]
- Höckel M, Knoop C, Schlenger K, et al. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol 1993;26:45-50. [PubMed]
- Halmos GB, de Bruin LB, Langendijk JA, et al. Head and neck tumor hypoxia imaging by 18F-fluoroazomycin-arabinoside (18F-FAZA)-PET: A review. Clin Nucl Med 2014;39:44-8. [PubMed]
- Bollineni VR, Kerner GS, Pruim J, et al. PET imaging of tumor hypoxia using 18F-fluoroazomycin arabinoside in stage III-IV non-small cell lung cancer patients. J Nucl Med 2013;54:1175-80. [PubMed]
- Rajendran JG, Mankoff DA, O’Sullivan F, et al. Hypoxia and glucose metabolism in malignant tumors: evaluation by (18F)fluoromisonidazole and (18F)fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res 2004;10:2245-52. [PubMed]
- Koh WJ, Bergman KS, Rasey JS, et al. Evaluation of oxygenation status during fractionated radiotherapy in human nonsmall cell lung cancers using (F-18)fluoromisonidazole positron emission tomography. Int J Radiat Oncol Biol Phys 1995;33:391-8. [PubMed]
- Koh WJ, Rasey JS, Evans ML, et al. Imaging of hypoxia in human tumors with (F-18)fluoromisonidazole. Int J Radiat Oncol Biol Phys 1992;22:199-212. [PubMed]
- Piert M, Machulla HJ, Picchio M, et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med 2005;46:106-13. [PubMed]
- Piert M, Machulla HJ, Becker G, et al. Dependency of the (18F)fluoromisonidazole uptake on oxygen delivery and tissue oxygenation in the porcine liver. Nucl Med Biol 2000;27:693-700. [PubMed]
- Airley RE, Loncaster J, Raleigh JA, et al. GLUT-1 and CAIX as intrinsic markers of hypoxia in carcinoma of the cervix: Relationship to pimonidazole binding. Int J Cancer 2003;104:85-91. [PubMed]
- Dubois L, Landuyt W, Haustermans K, et al. Evaluation of hypoxia in an experimental rat tumor model by [(18)F]fluoromisonidazole PET and immunohistochemistry. Br J Cancer 2004;91:1947-54. [PubMed]
- Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with (F-18)FLT and positron emission tomography. Nat Med 1998;4:1334-6. [PubMed]
- Tsuyoshi H, Morishita F, Orisaka M, et al. 18F-fluorothymidine PET is a potential predictive imaging biomarker of the response to gemcitabine-based chemotherapeutic treatment for recurrent ovarian cancer: Preliminary results in three patients. Clin Nucl Med 2013;38:560-3. [PubMed]
- Rasey JS, Grierson JR, Wiens LW, et al. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med 2002;43:1210-7. [PubMed]
- Yamamoto Y, Ono Y, Aga F, et al. Correlation of 18F-FLT uptake with tumor grade and Ki-67 immunohistochemistry in patients with newly diagnosed and recurrent gliomas. J Nucl Med 2012;53:1911-5. [PubMed]
- Pope WB, Kim HJ, Huo J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology 2009;252:182-9. [PubMed]
- Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 1999;9:53-60. [PubMed]
- Foroutan P, Kreahling JM, Morse DL, et al. Diffusion MRI and novel texture analysis in osteosarcoma xenotransplants predicts response to anti-checkpoint therapy. PLoS One 2013;8:e82875. [PubMed]
- Kummar S, Gutierrez ME, Chen A, et al. Phase I trial of vandetanib and bevacizumab evaluating the VEGF and EGF signal transduction pathways in adults with solid tumours and lymphomas. Eur J Cancer 2011;47:997-1005. [PubMed]
- Donaldson SB, Betts G, Bonington SC, et al. Perfusion estimated with rapid dynamic contrast-enhanced magnetic resonance imaging correlates inversely with vascular endothelial growth factor expression and pimonidazole staining in head-and-neck cancer: a pilot study. Int J Radiat Oncol Biol Phys 2011;81:1176-83. [PubMed]
- Takahashi R, Hirata H, Tachibana I, et al. Early (18F)fluorodeoxyglucose positron emission tomography at two days of gefitinib treatment predicts clinical outcome in patients with adenocarcinoma of the lung. Clin Cancer Res 2012;18:220-8. [PubMed]
- von Pawel J, von Roemeling R, Gatzemeier U, et al. Tirapazamine plus cisplatin versus cisplatin in advanced non-small-cell lung cancer: A report of the international CATAPULT I study group. Cisplatin and tirapazamine in subjects with advanced previously untreated non-small-cell lung tumors. J Clin Oncol 2000;18:1351-9. [PubMed]
- Rischin D, Hicks RJ, Fisher R, et al. Prognostic significance of (18F)-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: A substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol 2006;24:2098-104. [PubMed]
- Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial. Lancet Oncol 2007;8:797-805. [PubMed]
- Weber WA. Use of PET for monitoring cancer therapy and for predicting outcome. J Nucl Med 2005;46:983-95. [PubMed]
- Obrzut S, Bykowski J, Badran K, et al. Utility of fluorodeoxyglucose-positron emission tomography in the identification of new lesions in lung cancer patients for the assessment of therapy response. Nucl Med Commun 2010;31:1008-15. [PubMed]
- Zweifel M, Padhani AR. Perfusion MRI in the early clinical development of antivascular drugs: decorations or decision making tools? Eur J Nucl Med Mol Imaging 2010;37 Suppl 1:S164-82. [PubMed]
- Poncet B, Bachelot T, Colin C, et al. Use of the monoclonal antibody anti-HER2 trastuzumab in the treatment of metastatic breast cancer: A cost-effectiveness analysis. Am J Clin Oncol 2008;31:363-8. [PubMed]
- Jonker DJ, Rosen LS, Sawyer MB, et al. A phase I study to determine the safety, pharmacokinetics and pharmacodynamics of a dual VEGFR and FGFR inhibitor, brivanib, in patients with advanced or metastatic solid tumors. Ann Oncol 2011;22:1413-9. [PubMed]
- Wilson WH, Schenkein DP, Jernigan CL, et al. Reevaluating the accelerated approval process for oncology drugs. Clin Cancer Res 2013;19:2804-9. [PubMed]
- Van den Abbeele AD, Badawi RD. Use of positron emission tomography in oncology and its potential role to assess response to imatinib mesylate therapy in gastrointestinal stromal tumors (GISTs). Eur J Cancer 2002;38 Suppl 5:S60-5. [PubMed]
- Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using (18F)-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 1999;35:1773-82. [PubMed]
- Boellaard R, O’Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumor PET imaging: Version 1.0. Eur J Nucl Med Mol Imaging 2010;37:181-200. [PubMed]
- Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med 2006;47:1059-66. [PubMed]
- Clarke LP, Croft BS, Nordstrom R, et al. Quantitative imaging for evaluation of response to cancer therapy. Transl Oncol 2009;2:195-7. [PubMed]


