BRAF, MEK and KIT inhibitors for melanoma: adverse events and their management
Introduction
Targeted agents have rapidly become a standard in the therapy of various cancers. The mitogen activated protein kinase (MAPK) and PI3K/AKT pathway play a pivotal role in tumour development, growth and metastasis of melanoma. In cutaneous melanoma arising in non-chronically sun-damaged skin (nCSD), activating mutations frequently occur in the tyrosine kinase BRAF and, to a lesser degree in NRAS. In melanoma arising from CSD, mucosal, or acral surfaces, alterations in the type III transmembrane receptor tyrosine kinase KIT are more prevalent. These alterations, either as KIT mutations or gene amplifications, can lead to constitutive KIT activation and subsequently upregulation of the MAPK and PI3K/AKT pathway.
To this stage, two selective BRAF inhibitors, vemurafenib and dabrafenib, have been approved for the treatment of unresectable or metastatic melanoma harbouring activating mutations in BRAF by the Food and Drug Administration (FDA) in the USA and the European Medicines Agency (EMA). Other BRAF inhibitors are currently being tested in clinical trials (LGX818 developed by Novartis, NCT01436656). In 2013, trametinib, a small molecule blocking MEK1/MEK2, a tyrosine kinase downstream of BRAF, was first approved as a single agent by the FDA. Recently, accelerated approval was granted for the combination therapy of trametinib and dabrafenib due to the durable objective responses demonstrated in a randomized, multicentre, open-label study (1). Approval for the EU is expected for the first half of 2014. Several other MEK inhibitors have been or are under investigation in clinical trials (cobimetinib, Hoffmann-La Roche, NCT01689519; MEK162, Novartis, NCT01909453; selumetinib, AstraZeneca, NCT00866177). In contrast to the BRAF inhibitors, MEK inhibitors show activity in BRAF- and NRAS-mutant melanoma (2).
The targeted activity of these drugs allows to preselect patients likely to respond to treatment based on their tumour’s mutational profile and has led to unprecedented objective response rates (ORR) of approximately 50% and a progression free survival (PFS) time of 6-7 months of the BRAF inhibitors vemurafenib (3-5) and dabrafenib (6). The tumour response is lower in patients treated with single agent trametinib for both BRAF- and NRAS-mutant tumours (20% ORR) (2). Both substance classes are generally well tolerated but especially cutaneous toxicities are common for BRAF inhibitors because of paradoxical activation of the MAPK pathway in wild-type BRAF cells (7,8). Virtually all tumours will become resistant to selective inhibition with time. Both BRAF and MEK inhibitors have therefore been combined with different agents, the most successful treatment so far, however, was the combination with each other. In a phase I/II trial, 150 mg of the BRAF inhibitor dabrafenib plus 2 mg/d of the MEK inhibitor trametinib resulted in a 76% ORR and impressive durable responses (1). Additionally, the adverse event (AE) profile changed with less proliferative skin lesions but a higher rate of pyrexia.
For melanoma with molecularly proven somatic alterations in KIT, three clinical trials of the multikinase inhibitor imatinib were conducted, which showed ORR of 16-25% (9-11) with durable responses (10). Patients who responded generally harboured KIT mutations involving exon 11 or 13, especially L576P (exon 11) or K642E (exon 13) mutations (12). Response to KIT inhibition has also been reported for other targeted inhibitors such as dasatinib (13) and sunitinib (14). So far, sunitinib (15), dasatinib (16) and nilotinib have been (17) used in clinical trials. Due to the comparable rarity of KIT mutations in melanoma, total accrual and completion of clinical trials with KIT inhibitors has proven to be challenging. Results of the phase 2 study (TEAM trial, NCT01028222) specifically for KIT mutated melanoma investigating nilotinib are still not published.
The experience that has been gained with the use of targeted inhibitors in recent years has led to the identification of a class specific AE profile for these drugs. In this review, the unique AEs will be highlighted and—where known—treatment recommendations given.
BRAF inhibitors
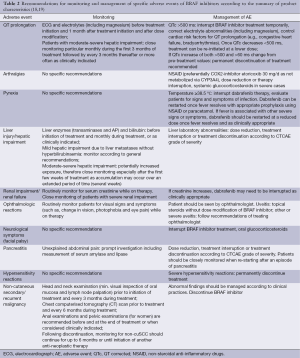
The majority of patients (>90%) experience AEs, however, these are generally mild to moderate (Common Toxicity Criteria of Adverse Events, CTCAE v4.0, grade 1-2) (4-6). In patients with grade 1 and tolerable grade 2 toxicities, treatment can be continued at the usual dosage of 960 mg twice daily (BD) for vemurafenib and 150 mg BD for dabrafenib according to the summary of product characteristics (18,19). Patients experiencing intolerable grade 2 or 3 toxicity should interrupt therapy until toxicity is grade 0-1 and reduce by one dose level when therapy is being resumed. Patients with grade 4 toxicity should discontinue permanently, or interrupt therapy until grade 0-1 and reduce by two dose levels for vemurafenib and one dose level for dabrafenib when resuming therapy. Patients with a QTc time >500 ms should not receive vemurafenib. Additional information regarding dose reduction with recurring toxicity and occurence of prolonged QTc times can be found in the respective summary of product characteristics.
General adverse events (AEs)
Frequent non-cutaneous AEs include arthralgia, fatigue, nausea, diarrhea and headache. AEs that occurred in >10% of patients in the major studies of vemurafenib and dabrafenib are listed in Table 1. Important AEs will be discussed in the text, monitoring and management recommendations are given in Table 2.
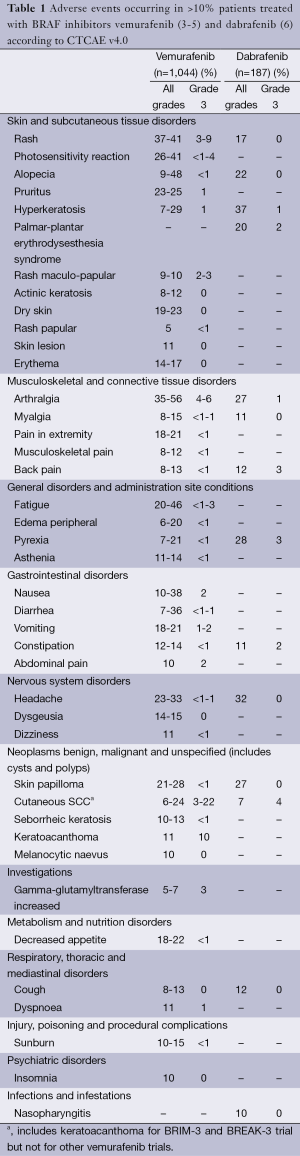
Full table
Full table
QT prolongation
QT prolongation has been observed in both vemurafenib and dabrafenib and was shown to be exposure-dependent (19). As QT prolongation can lead to an increased risk of ventricular arrhythmias, including Torsades de Pointes, treatment with either BRAF inhibitor is not recommended in patients with uncorrectable electrolyte abnormalities (including magnesium), long QT syndrome or who are taking medicinal products known to prolong the QT interval (18,19).
Arthralgias
Arthralgias are frequent during treatment with selective BRAF inhibitors, patients reported arthralgia in 35-56% (4-6% grade 3, no grade 4 toxicities) for vemurafenib (3-5) and 27% (1% grade 3, no grade 4 toxicities) for dabrafenib (6). They usually affect finger, hand, elbow, knee and ankle joints, concomitant panniculitis has been described (20). Arthralgia can usually be treated with nonsteroidal anti-inflammatory drugs (NSAID). However, as many NSAID are metabolized via the cytochrome (CYP)-P450 isoenzyme 2C9, the bioavailability of selective BRAF inhibitors can be markedly affected. Etoricoxib, a COX-2 inhibitor can be administered, which is only partly metabolized via CYP3A4 and which in our experience is effective and well-tolerated in a dose of 30 mg daily (20). In cases of severe arthralgia, a dose reduction or therapy interruption may be necessary.
Pyrexia
Pyrexia occurred in 28% of dabrafenib-treated patients in the phase III study (6) and in 7-21% of vemurafenib-treated patients (3-5). No grade 3-4 pyrexia was reported for either BRAF inhibitor in these studies. In 1% of dabrafenib-patients in clinical trials, serious non-infectious febrile events defined as fever accompanied by severe rigors, dehydration, hypotension and/or acute renal insufficiency of pre-renal origin in subjects with normal baseline renal function were identified. Onset of these serious non-infectious febrile events was typically within the first month of therapy and patients responded well to dose interruption and/or dose reduction and supportive care. The mechanism of dabrafenib-induced pyrexia is unclear, although it is postulated that dabrafenib can cause a potent inflammatory response that leads to fever in certain patients.
Liver injury/hepatic impairment
Liver laboratory abnormalities especially increase of GGT, alkaline phosphatase (AP), ALT and bilirubin may occur with vemurafenib. No adjustment to the starting dose is needed for patients with hepatic impairment for both vemurafenib and dabrafenib (18,19). Special caution may be warranted in patients receiving radiotherapy in whom the radiotherapy portal includes the liver as one case of severe radiation-induced liver toxicity in a young female patient treated with vemurafenib for metastatic melanoma was reported (21). The patient developed severe zone III necrosis and scattered venous thrombi as well as a hepatic haematoma and haemoperitoneum consistent with hepatic haemorrhage weeks after radiotherapy of bone metastases and cauda equina compression due to metastasis.
Renal impairment/renal failure
As hepatic metabolism is the main route of drug excretion, BRAF inhibitors are relatively safe for the kidneys. Renal failure has been identified in <1% of patients treated with dabrafenib. It was generally associated with pyrexia and dehydration and responded well to dose interruption and general supportive measures. Granulomatous nephritis has been reported (18).
Regnier-Rosencher et al. (22) described the occurrence of acute kidney injury with severe grade 3 cutaneous reactions and partially high eosinophil counts in four elderly patients within 14 days after starting vemurafenib treatment for metastatic melanoma. They speculated that the underlying cause was an acute immunoallergic interstitial nephritis induced by vemurafenib. Interestingly, all patients showed major or complete tumour responses.
Both BRAF inhibitors have not been studied in patients with severe renal impairment, therefore caution should be used and patients closely monitored. There is one report of a patient with end-stage renal failure on continuous ambulatory peritoneal dialysis who received 960 mg vemurafenib BD for metastatic melanoma (23). His renal function and electrolytes remained stable under treatment with vemurafenib, but he developed an increased QTc interval >500 ms five months after treatment initiation. After pausing vemurafenib for three weeks, QTc returned to baseline and the patient continued with a reduced dose (720 mg BD).
Ophthalmologic reactions
Serious ophthalmologic reactions, including uveitis (5,24-26), iritis and retinal vein occlusion (27), have been reported for both BRAF inhibitors. Uveitis can generally be treated with topical corticosteroids while continuing BRAF-inhibitor treatment. However, in one case vemurafenib induced pan-uveitis led to near-complete visual loss (24).
Neurological symptoms
Klein et al. (27) report on three patients with idiopathic facial palsy occurring while under treatment with vemurafenib at their institution (2-9 months after treatment initiation). As facial palsy was synchronously bilateral in two of the patients and no known causes associated with facial palsy could be identified in any patient, the authors speculate that RAF inhibitor–induced paradoxical activation of the MAPK pathway, with subsequent proliferation of Schwann cells, as observed in rat toxicology studies with a different RAF inhibitor (28) might be a possible underlying mechanism. Alternatively, immunologic mechanisms may have been involved. Treatment with oral glucocorticosteroids led to a complete resolution of deficits in two of the patients.
Pancreatitis
Pancreatitis has been reported in <1% of dabrafenib-treated patients. One of the events occurred on the first day of dosing and recurred following re-challenge at a reduced dose (18). In a vemurafenib-treated patient pancreatitis occurred two weeks after treatment initiation (29) and re-challenge with a reduced vemurafenib dose led to exacerbated symptoms of pancreatitis after two doses.
Hypersensitivity reaction
Serious hypersensitivity reactions, including anaphylaxis have been reported in association with vemurafenib. Severe hypersensitivity reactions may include Stevens-Johnson syndrome, generalized rash, erythema or hypotension.
Non-cutaneous secondary/recurrent malignancy
Cases of non-cutaneous squamous cell carcinoma (non-cu SCC) have been reported in clinical trials where patients received either BRAF inhibitor. In vitro experiments have demonstrated paradoxical activation of MAPK signaling in BRAF wild type cells with RAS mutations when exposed to BRAF inhibitors (7,8,30,31). This may lead to increased risk of non-cutaneous malignancies with BRAF-inhibitor exposure when RAS mutations are present. Cases of RAS-associated malignancies have been reported with vemurafenib [chronic myelomonocytic leukemia (CMML) (32) and non-cutaneous SCC of the head and neck] and with dabrafenib when administered in combination with the MEK inhibitor, trametinib (colorectal cancer and glioblastoma) (33). A thorough work-up of the patient developing CMML showed vemurafenib dose-dependent, reversible activation of ERK in the NRAS-mutated leukemic clones while vemurafenib caused regression of the patient’s BRAF V600K—mutant melanoma (32). The authors assume the preexistence of a clinically undiagnosed NRAS-mutant leukemic clone, which was specifically induced to proliferate on treatment with vemurafenib. In consequence, BRAF inhibitors should not be used in patients with known RAS-mutant tumor in the history.
Cutaneous adverse events (AEs)
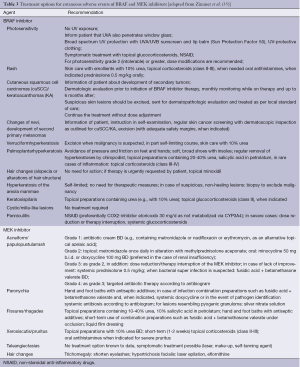
Almost all patients with BRAF inhibitor therapy will experience at least one dermatologic AE. In an analysis of patients treated across vemurafenib clinical trials, dermatologic AEs were documented in 92-95% of patients (34). Cutaneous AEs are mostly grade 1-2 in severity and symptomatic treatment to alleviate discomfort generally suffices. Rash, hyperkeratotic lesions, photosensitivity, alopecia, hand-foot syndrome and keratoacanthoma/squamous cell carcinoma are commonly reported. Table 3 lists common cutaneous AEs and treatment options.
Full table
Rash
In the study by Lacouture et al. (34), rash incidence ranged from 64-75%. Rash can be macular-papular or more verrucous and hyperkeratotic, by some authors described as Darier- or Morbus Grover-like due to the dyskeratosis and acantholysis it displays on histopathologic examination (36,37). In the latter case, especially the ventral and dorsal midline of the trunk as well as the legs are affected. The occurrence or severity of rash did not appear to be associated with treatment response (34). Rash can generally be treated symptomatically, dose modifications and interruptions are rarely necessary.
Treatment recommendations for rash are based on the experience of the authors in managing patients, and are similar to those used in general dermatology practice (Table 3).
Keratoacanthoma (KA) and cutaneous squamous cell carcinoma (cuSCC)
KA and cuSCC have been reported in 20-30% of vemurafenib-treated patients (4,34) and in 10-20% of dabrafenib-treated patients and develop within 2-36 weeks after initiation of therapy especially on sun-exposed skin. The cuSCC are normally well-differentiated and both KA and cuSCC do not require further therapy other than complete excision. The pathogenesis is not fully understood but molecular genetic studies of SCC that developed during BRAF inhibitor therapy showed an increased rate of RAS mutations, particularly H-RAS mutations (38,39). Results of research on the paradoxical activation of MAPK by RAF inhibitors predicts that upstream oncogenic events, either activating mutations in RAS or mutations or amplifications in receptor tyrosine kinases that strongly elevate levels of the RAS-guanosine triphosphate complex in the absence of a BRAF V600E mutation, would potentiate signaling through the MAPK pathway (7,8,30). There is increasing evidence that the development of cuSCC and KA in BRAF inhibitor treated patients may arise from paradoxical MAPK-pathway activation.
Other hyperkeratotic lesions, including verruca vulgaris, milia, actinic keratoses and multiple ‘keratotic warty papules’, are commonly reported, suggesting a spectrum of proliferative lesions of keratinocytes from hyperkeratosis and keratosis pilaris, to KA and cuSCC (Figure 1) (34). As many of these lesions are often associated with HPV infection and as HPV infection can be pathogenic in other squamous malignancies, the role of HPV for the development of cuSCC and KA has been discussed. An analysis of lesions collected from dabrafenib-treated patients suggested no association between high-risk HPV and the development of keratotic proliferative lesions, including SCC (40). However, there is experimental evidence that vemurafenib cooperates with HPV to initiate cutaneous squamous cell carcinomas (41).
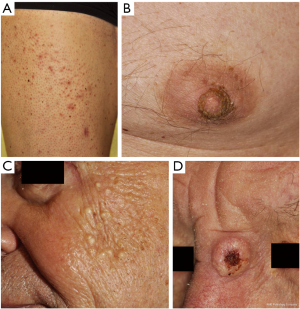
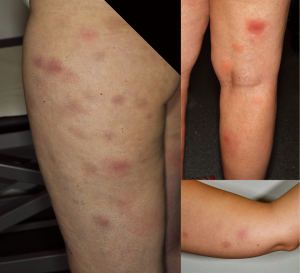
Photosensitivity
Mild to severe photosensitivity (Figure 2) has been reported frequently in patients receiving vemurafenib (26-37%) (4,5,42) but not dabrafenib and can pose a significant problem for patients as they are limited in activities of daily life even when applying sunscreen or on cloudy days. Severe sunburn reactions with painful blistering have been described and patients have to be advised that reactions can occur even through glass as the phototoxicity is mainly UVA-dependent (43).
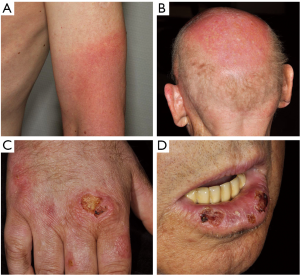
Panniculitis
Panniculitis (Figure 3) as well as erythema nodosum-like lesions have been described for both vemurafenib and dabrafenib (20,40,44-48). In their prospective study, Boussemart et al. (47) reported a panniculitis frequency of 14% in vemurafenib-treated patients. Management should be adjusted to the symptom severity and presence of arthralgias. Treatment interruption and dose reduction are not always required and spontaneous resolution has been described (46). In one case presented by us (20), early intervention with treatment discontinuation, systemic glucocorticosteroids as well as COX-2 inhibitors and dose reduction once symptoms had improved showed beneficial.
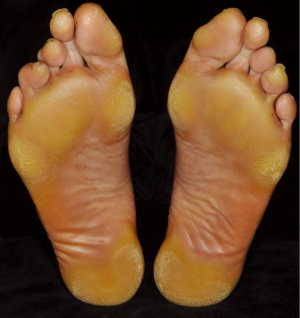
Plantar hyperkeratosis
Hyperkeratosis primarily affecting areas of friction on the soles of the feet (Figure 4) occurs commonly even though rarely reported in the large clinical trials. For vemurafenib, plantar hyperkeratosis was reported in 6-7% of patients (4,42). Boussemart et al. (47), however, noted mild forms of hand-foot skin reaction in 60% of their patients. It usually presents as localized yellowish, hyperkeratotic plaques in areas under physical pressure. The callus like palmo-plantar hyerkeratosis without significant inflammation must be carefully distinguished from the plamo-plantar dysesthesia syndrome (49) seen under multikinase inhibitors such as sorafenib. Unlike sorafenib, severe reactions with bullous lesions are rare and patients are normally not limited in the activities of daily life.
Hair changes
Mild alopecia was reported in 35% of patients in the BRIM3 trial and in 12% of patients in the phase II trial of dabrafenib. With duration of treatment, most patients will experience alterations of hair growth, mostly slower growth of both scalp and body hair. Some patients note a wavy, curly hair structure. There is no proven treatment for alopecia, but patients can try topical minoxidil.
New (eruptive) naevi and primary melanoma
Changes of existing naevi as well as the development of new naevi and second primary melanomas have been described for both vemurafenib and dabrafenib (6,42,50-54). Interestingly, the new lesions are generally BRAF-wildtype, some even displaying NRAS mutations (54). Even though it is a well-known fact that patients with a history of melanoma have a higher risk of a second melanoma, the frequency seems to be considerably higher in patients undergoing BRAF inhibitor treatment (50,55). Similar to the development of cuSCC, a paradoxical activation of the MAPK pathway is thought to be the causing factor. Monitoring for skin lesions should occur as outlined for cuCSS and suspicious lesion excised while patients continue treatment without dose adjustment.
Severe dermatologic reactions
Severe dermatologic reactions have been reported in patients receiving vemurafenib, including rare cases of Stevens-Johnson syndrome and toxic epidermal necrolysis (56), drug reaction with eosinophilia and systemic symptoms (DRESS) (57) and sweet syndrome (58). In patients who experience a severe dermatologic reaction, vemurafenib treatment should be permanently discontinued. Re-induction using a different BRAF-inhibitor has successfully been done.
Radiosensitization and radiation recall induced by BRAF inhibitors
Several authors have described an increased radiosensitivity and radiation recall in patients under BRAF inhibitor therapy (21,59-62). Severe radiotherapy-induced toxicity can affect the skin was well as extracutaneous sites (21,61). Dose reduction or interruption should therefore be discussed in patients undergoing radiotherapy and patients closely monitored for signs of radiotoxicity. In our own experience, radiotherapy of the brain, especially stereotactic therapy is well tolerated and does not require dose adjustments of either radiotherapy or BRAF inhibitor therapy (63). Harding et al. (64), however, reported on two patients who developed an eruption of the scalp termed cutis verticis gyrata in context of vemurafenib and whole brain radiotherapy (WBRT). The first patient received vemurafenib and WBRT concomitantly, whereas the second patient started treatment with vemurafenib three weeks after the completion of WBRT.
MEK inhibitors
Trametinib is currently the only MEK inhibitor approved by the FDA. Recommended dose modifications for specific AEs are therefore currently only available for trametinib (Table 4), for all other MEK inhibitors, dose modifications should be handled as per the relevant clinical trial protocol. Recommended dose for trametinib is 2 mg once daily until disease progression or unacceptable toxicity.
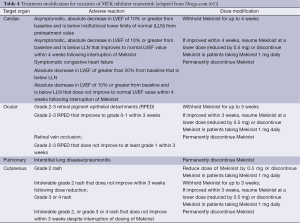
Full table
General adverse events (AEs)
The most common general side effects of MEK inhibitors are diarrhea, peripheral edema, fatigue, nausea and vomiting (66-68). AEs that occurred in >10% are listed in Table 5. Important AEs will be discussed in the text.
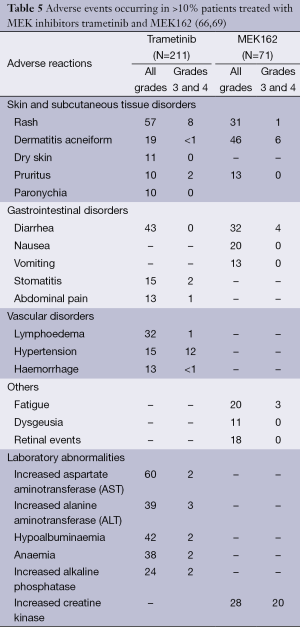
Full table
Cardiomyopathy
Cardiomyopathy [defined as cardiac failure, left ventricular dysfunction, or decreased left ventricular ejection fraction (LVEF)] occurred in 7% of patients treated with trametinib in the phase I study (70). The median time to onset of cardiomyopathy was 63 days (range: 16 to 156 days); in five patients cardiomyopathy was identified within the first month (71). Asymptomatic decreases of LVEF were seen in 3 of 71 patients in the phase II trial of MEK162 (66) and 3 of 15 patients treated with selumetinib experienced cardiac dysfunction, 2 of these were grade III in severity (68).
LVEF should be determined by echocardiogram or multigated acquisition (MUGA) scan before initiation, one month after treatment initiation and then at 2- to 3-month intervals while on treatment.
Ocular toxicities
Ocular toxicities such as blurred vision, macula edemas, retinal pigment epithelial detachment (RPED), retinal vein occlusion (RVO), central serous retinopathy, glaucoma and elevated intraocular pressure can occur. RPED and RVO are severe events requiring urgent intervention. The incidence of RPED across all clinical trials of trametinib was 0.8% (14 of 1,749 patients) (71). Retinal detachments were often bilateral and multifocal, occurring in the macular region of the retina. The incidence of RVO was 0.2% across all trials (71). An RVO may lead to macular edema, decreased visual function, neovascularization, and glaucoma. In the phase 2 trial of MEK162, retinal events (including retinal detachment, retinal pigment epithiolopathy, retinoschisis, retinal oedema, chorioretinopathy, retinopathy and retinal exudates) occurred in 13 of 71 patients (18.3%). None of the events were grade 3 or 4 in severity, transient in nature and resolved without treatment discontinuation, after dose reduction or after interruption of treatment. Ophthalmological evaluation should be performed at any time a patient reports visual disturbances and compared to baseline.
Interstitial lung disease (ILD)
ILD or pneumonitis occurred in 1.8% of patients across all clinical trials of trametinib generally requiring hospitalization (71). The median time to first presentation of ILD or pneumonitis was 160 days (range: 60 to 172 days) (71).
Trametinib should be withheld in patients presenting with new or progressive pulmonary symptoms and findings including cough, dyspnea, hypoxia, pleural effusion, or infiltrates, pending clinical investigations and permanently discontinued for patients diagnosed with treatment-related ILD or pneumonitis (71).
Creatine kinase (CK) elevations
Increases of CK were among the most prevalent treatment-related AEs of MEK162 and constituted the most common grade 3-4 toxicity even though they were mostly asymptomatic (66). Symptoms of CK increase include muscle weakness and myalgia. CK was not measured in trametinib trials. The true significance especially of asymptomatic CK increases is still unknown, but patients should be closely monitored and dosage decreased or interrupted as per protocol or severity of toxicity. Interestingly, Moreno Garcia et al. (72) found an association between CK elevation and skin rash of novel targeted agents in phase I trials and hypothesized that these were due to increased CK-BB (brain and skin) expression of keratinocytes.
Neurological symptoms
Chen et al. (73) reported on three patients treated with selumetinib who developed a “dropped head syndrome”, an uncommon progressive weakness of neck extensor muscles. One other case was reported for a patient treated with PD-0325901, another MEK inhibitor (74). The dropped head syndrome is clinically characterized by a focal noninflammatory myopathy, moderately elevated CK, no response to corticosteroids and a full recovery after discontinuation of the offending agent.
Cutaneous adverse events (AEs)
Cutaneous reactions under MEK inhibitors occur commonly, 87% of patients treated with trametinib experienced some form of skin toxicity (71). The clinical spectrum resembles the known cutaneous toxicities that can occur during therapy with epidermal growth factor receptor (EGFR) inhibitors. In contrast to BRAF inhibitors, an increased rate of cuSCC or KA has not been noted.
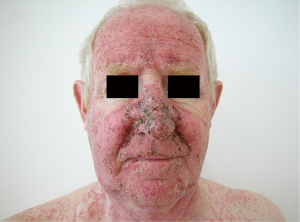
The acneiform or papulopustular rash usually appears in the first three weeks after therapy initiation in the seborrheic areas such as face and trunk (Figure 5). After therapy discontinuation it is usually completely reversible within 4-6 weeks (75,76). Bacterial superinfection with Staphylococcus aureus may occur (75). Other cutaneous lesions that are usually observed after more than six weeks of therapy are paronychia, xerosis cutis, pruritus, fissures of the finger tips and heels, cheilitis angularis, alopecia, teleangiectasia and hyperpigmentation (75,76). Schad et al. (76) additionally reported depigmentation of scalp hair as has already been observed in therapy with c-Kit inhibitors (77). After interruption of therapy, re-pigmentation occurred regularly. Pathophysiologically, inhibition of the MAPK-signaling pathway in keratinocytes appears to result in similar cutaneous side effects either by blockade of the EGF receptor or the MEK kinase (76). Therefore, the same recommendations as for EGFR-induced cutaneous lesions apply for the prevention and therapy of cutaneous side effects during MEK inhibitor therapy (Table 3). Severe skin toxicity occurred in 12% of patients treated with trametinib often due to secondary infection (71).
Combination of BRAF and MEK inhibitors
In the phase I/II trial of combined treatment with dabrafenib and trametinib, grade 3-4 events occurred in 58% of patients treated with the full dose combination therapy (150 mg dabrafenib and 2 mg trametinib) compared to 43% of patients treated with single agent dabrafenib (1). The most frequent AEs observed in the 150/2 combination group were pyrexia (71%), chills (58%) fatigue (53%), nausea (44%), vomiting (40%), and diarrhea (36%). The most frequently occurring grade 3 or 4 toxic effect was neutropenia (in 11% of patients), with one case of febrile neutropenia.
The incidence of acneiform dermatitis was reduced in the combination therapy and the rate of cuSCC was lower for the combination treatment compared to dabrafenib alone (7% vs. 19%) as was expected (1). A report of sarcoidal granulomatous inflammation in one patient with asymptomatic, self-limited papular eruptions and in another patient within a junctional naevus adds to the spectrum of cutaneous AEs (78).
KIT inhibitors
As the multikinase inhibitors imatinib, sunitinib, dasatinib and nilotinib block different kinases apart from KIT, their AE profile differs partially from each other. None of these drugs have been FDA- or EMA-approved for the treatment of melanoma, therefore no general dosing or dose reduction recommendations can be given and patients should preferentially be treated within clinical trials. Most experience on AEs has been gained within clinical trials for malignancies other than melanoma. As these diseases differ substantially from melanoma, observed adverse reaction rates cannot be directly translated to melanoma.
General adverse events (AEs)
Myelosuppression, fatigue, fluid retention and QT prolongation are frequently observed adverse reactions for most KIT inhibitors and are generally well tolerated by melanoma patients (9-11,13,15-17). Dose reductions were required in 46% patients treated with imatinib (10), but rarely for the other KIT inhibitors.
Myelosuppression
Thrombocytopenia, neutropenia, and anemia are frequent in patients treated with imatinib (79), dasatinib (80) and nilotinib (81) and can be severe (grade 3 or 4). They generally occur in the first months of treatment.
For dasatinib, complete blood counts should be performed weekly for the first two months and then monthly thereafter, or as clinically indicated. For nilotinib, intervals should be every two weeks for the first two months and then monthly thereafter, or as clinically indicated. Myelosuppression was generally reversible and usually manageable by withholding dasatinib temporarily or by dose reduction.
Oedema and fluid retention
Imatinib and dasatinib are often associated with oedema and occasionally serious fluid retention in up to 10% of patients (79,80). Severe ascites, pulmonary edema, and generalized oedema have been reported occasionally. In the phase 2 trial of dasatinib in melanoma, 47% developed pleural effusion with 9% grade 3-4 toxicity (16).
Patients should be weighed and monitored regularly for signs and symptoms of fluid retention. Patients who develop symptoms suggestive of pleural effusion, such as dyspnea or dry cough, should be evaluated by chest X-ray. Fluid retention events were typically managed by supportive care measures that include diuretics or short courses of steroids.
Hepatic impairment/hepatotoxicity
Sunitinib has been associated with hepatotoxicity, which may result in liver failure (0.3% in clinical trials and post-marketing experience) or death (82). Liver function tests (ALT, AST, bilirubin) should be monitored before initiation of sunitinib treatment, during each cycle of treatment and as clinically indicated, and sunitinib interrupted for grade 3-4 drug-related hepatic AEs and discontinued if there is no resolution. Sunitinib should not be restarted if patients subsequently experience severe changes in liver function tests or have other signs and symptoms of liver failure. Safety in patients with ALT or AST >2.5× ULN or, if due to liver metastases, >5.0× ULN has not been established.
Cases of fatal liver failure and severe liver injury requiring liver transplants have been reported with both short-term and long-term use of imatinib (79). Liver function (transaminases, bilirubin, and AP) should be monitored before initiation of treatment and monthly, or as clinically indicated. Laboratory abnormalities should be managed with imatinib interruption and/or dose reduction.
As nilotinib exposure is increased in patients with impaired hepatic function, a lower starting dose for patients with mild to severe hepatic impairment (at baseline) should be used and the QT interval monitored frequently (81).
Cardiotoxicity
Cardiac adverse reactions were reported in 7% of 258 patients taking dasatinib, including cardiomyopathy, congestive heart failure (CHF), diastolic dysfunction, fatal myocardial infarction, and left ventricular dysfunction (LVD) (80). Therefore, patients should be monitored for signs or symptoms consistent with cardiac dysfunction and treated appropriately.
Severe CHF and LVD have also been reported in patients taking imatinib (79). Most patients have had other co-morbidities and risk factors, including advanced age and previous medical history of cardiac disease. Patients with cardiac disease or risk factors for cardiac or history of renal failure should be monitored carefully and any patient with signs or symptoms consistent with cardiac or renal failure should be evaluated and treated.
For sunitinib, cardiovascular events, including heart failure, myocardial disorders and cardiomyopathy, some of which were fatal, have been reported (82). Patients who presented with cardiac events within 12 months prior to sunitinib administration were excluded from sunitinib clinical studies. It is unknown whether patients with these concomitant conditions may be at a higher risk of developing drug-related LVD. These patients should be carefully monitored for clinical signs and symptoms of CHF while receiving sunitinib. Baseline and periodic evaluations of LVEF should be considered. In patients without cardiac risk factors, a baseline evaluation of ejection fraction should be considered. In the presence of clinical manifestations of CHF, discontinuation of sunitinib is recommended and sunitinib should be interrupted and/or reduced in patients without clinical evidence of CHF but with an ejection fraction <50% and >20% below baseline.
QT interval prolongation
Sunitinib and nilotinib have been shown to prolong the QT interval in a dose dependent manner, which may lead to an increased risk for ventricular arrhythmias including Torsades de Pointes (81,82). Sunitinib and nilotinib should be used with caution in patients with a history of QT interval prolongation, patients who are taking antiarrhythmics, or patients with relevant pre-existing cardiac disease, bradycardia, or electrolyte disturbances. Periodic monitoring with on-treatment electrocardiograms and electrolytes (magnesium, potassium) should be considered. Concomitant treatment with strong CYP3A4 inhibitors, which may increase sunitinib and nilotinib plasma concentrations, should be used with caution and dose reduction of sunitinib should be considered. For nilotinib, inappropriately intake with food can also lead to an increased plasma concentration.
In vitro data suggest that dasatinib has the potential to prolong the QT interval, but in clinical trials, QTc prolongation was reported in only 1% of patients (80). Caution should be used in patients who have or may develop prolongation of QTc for both dasatinib and imatinib (79,80).
Hypertension
A total of 34% of patients receiving sunitinib for treatment-naïve renal cell carcinoma experienced hypertension, in 13% severity was grade 3 (82). Patients should be monitored for hypertension and treated as needed with standard anti-hypertensive therapy. In cases of severe hypertension, temporary suspension of sunitinib is recommended until hypertension is controlled.
Dasatinib may increase the risk of developing pulmonary arterial hypertension (PAH) which may occur any time after initiation, including after more than one year of treatment (80). Manifestations include dyspnea, fatigue, hypoxia, and fluid retention. PAH may be reversible on discontinuation. Patients should be evaluated for signs and symptoms of underlying cardiopulmonary disease prior to initiating dasatinib and during treatment. If PAH is confirmed, dasatinib should be permanently discontinued.
Haemorrhagic events
In patients receiving sunitinib, haemorrhagic events, some of which were fatal, have included gastrointestinal, respiratory, tumour, urinary tract and brain haemorrhages (82). These events may occur suddenly, and in the case of pulmonary tumours may present as severe and life-threatening haemoptysis or pulmonary haemorrhage. Serious, sometimes fatal gastrointestinal complications including gastrointestinal perforation, have occurred rarely in patients with intra-abdominal malignancies treated with sunitinib. Clinical assessment of these events should include serial complete blood counts and physical examinations.
In all clinical studies, severe central nervous system (CNS) haemorrhages, including fatalities, occurred in 1% of patients receiving dasatinib (80). Severe gastrointestinal haemorrhage, including fatalities, occurred in 4% of patients and generally required treatment interruptions and transfusions. Other cases of severe haemorrhage occurred in 2% of patients. Most bleeding events were associated with severe thrombocytopenia. Caution is warranted if patients are required to take medications that inhibit platelet function or anticoagulants and platelet count should be >50,000-75,000/µL.
Haemorrhages have also been frequently reported for imatinib (79). As gastrointestinal tumour sites may be the source, patients should be monitored for gastrointestinal symptoms at the start of therapy.
Thyroid dysfunction
Cases of hyperthyroidism, some followed by hypothyroidism, have been reported for sunitinib (82). Baseline laboratory measurement of thyroid function is recommended and patients with hypothyroidism or hyperthyroidism should be treated as per standard medical practice prior to the start of sunitinib treatment. All patients should be observed closely for signs and symptoms of thyroid dysfunction, including hypothyroidism, hyperthyroidism, and thyroiditis.
Clinical cases of hypothyroidism have been reported in thyroidectomy patients undergoing levothyroxine replacement during treatment with imatinib (79). TSH levels should be closely monitored in such patients.
Dermatologic toxicities
Cutaneous reactions, especially rash, pruritus and alopecia occur commonly with multikinase inhibitors and are generally dose-dependent. Non-specific, generally self-limiting skin rashes have been reported in 30-40% of CML patients treated with imatinib (77,83). There are some reports about classic drug-associated skin manifestations, such as vasculitis, Stevens-Johnson syndrome, acute generalized exanthematous pustulosis, oral lichenoid reaction, Sweet syndrome and toxic epidermal necrosis with imatinib, dasatinib and nilotinib (77,83). Upon re-challenge with imatinib, a recurrent dermatologic reaction was observed in some patients, in others, a lower dose did not lead to a flare-up of symptoms. Concomitant treatment with corticosteroids or antihistamines should be considered on re-challenge.
Acral erythema/hand-foot syndrome
Hand-foot syndrome commonly induced by sunitinib is similar to the palmoplantar reactions BRAF inhibitors can cause. It manifests as acral erythema with painful symmetric erythematous and oedematous areas on the palms and soles, often preceded or accompanied by paraesthesias (tingling sensations and intolerance to contact with hot objects) that are aggravated by warm environments (77). It generally arises after 2-4 weeks of treatment and seems to be dose dependent. Prophylaxis as well as treatment recommendations are the same as for HFS reaction of BRAF inhibitors (Table 3).
Subungual splinter haemorrhages
Painless distal subungual splinter haemorrhages under the fingernails and less commonly under the toenail scan be noted in patients using sunitinib and rarely with imatinib (77). They are thought to be thrombotic or embolic in origin. No prophylaxis or treatment is required.
Modification of hair and skin pigmentation
Hair depigmentation can be seen in patients after 5-6 weeks’ treatment with sunitinib, but the effect is reversible as early as 2-3 weeks after treatment discontinuation (77). It is thought to be caused by blockade of stem-cell-factor or c-KIT signalling. For imatinib, paradoxically, both skin and hair depigmentation (84,85), but conversely, hair re-pigmentation and hyperpigmentation of the skin has been described (85,86).There are also several cases of increased or reduced photosensitivity for imatinib (77,83). Skin and hair depigmentation is a rare event for dasatinib (83).
Periocular oedema
The periocular oedema commonly caused by imatinib is particularly visible in the periorbital area. In two prospective studies, superficial oedema was reported at a rate of 48-65% occurring at an average of six weeks after drug initiation (85,87). Imatinib treatment gives rise to epiphora or to more severe ophthalmological symptoms, such as visual obstruction due to intense eyelid oedema, extraocular muscle palsy, ptosis, blepharoconjunctivitis, visual obstruction, or evenretinal oedema (77). Mild to moderate facial oedema, mainly on the eyelids without any weight gain is seen in about 50% of patients given sunitinib (77). Moderate periorbital oedema does not generally need treatment. More severe ocular side-effects should be managed by ophthalmologists (88).
Discussion
Tyrosine kinase inhibitors have shown impressive clinical responses in advanced melanoma, however, display a unique array of AEs. A sound knowledge of these potential toxicities is important for adequate AE management and for prevention of unnecessary dose reductions or interruptions. The impending risk for the development of second primary tumours that have been noted under monotherapy with BRAF inhibitors might limit their use in the adjuvant setting.
Acknowledgements
Disclosure: (I) Elisabeth Livingstone: Consultancies and Honoraria: Bristol Myers Squibb GmbH & Co KG, Munich; Boehringer-Ingelheim Pharma GmbH & Co KG, Ingelheim; Speakers bureau: Amgen GmbH, Munich; Boehringer Ingelheim Pharma GmbH & Co KG, Ingelheim; Roche GmbH, Grenzach-Wyhlen; Bristol-Myers Squibb GmbH & Co KG, Munich; MSD SHARP & DOHME GmbH, Haar; Merck KGaA, Darmstadt. (II) Dirk Schadendorf: Consultancies, Speakers Bureau, Honoraria or travel support from Bristol Myers Squibb GmbH & Co KG, Munich; Boehringer-Ingelheim Pharma GmbH & Co KG, Ingelheim; Amgen GmbH, Munich; Roche GmbH, Grenzach-Wyhlen; MSD SHARP & DOHME GmbH, Haar; GlaxoSmithKline GmbH & Co. KG, Munich; Novartis, Nuremberg. (III) Julia Vaubel: Consultancies, Speakers Bureau, Honoraria or travel support from Bristol Myers Squibb GmbH & Co KG, Munich; Boehringer-Ingelheim Pharma GmbH & Co KG, IngelheimAmgen GmbH, Munich; Roche GmbH, Grenzach-Wyhlen; MSD SHARP & DOHME GmbH, Haar; Merck KGaA, Darmstadt; GlaxoSmithKline GmbH & Co. KG, Munich; Galderma Laboratorium GmbH, Düsseldorf; Cephalon GmbH, Munich; Teva GmbH, Berlin. (IV) Lisa Zimmer: Consultancies, Speakers Bureau, Honoraria and travel support: Bristol Myers Squibb GmbH & Co KG, Munich; Roche GmbH, Grenzach-Wyhlen; Novartis Pharma GmbH, Nürnberg;GlaxoSmithKline GmbH & Co. KG, Munich; Boehringer-Ingelheim Pharma GmbH & Co KG, Ingelheim; Amgen GmbH, Munich; Merck KGaA, Darmstadt.
References
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [PubMed]
- Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 2013;14:249-56. [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [PubMed]
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15:323-32. [PubMed]
- Flaherty L, Hamid O, Linette G, et al. A single-arm, open-label, expanded access study of vemurafenib in patients with metastatic melanoma in the United States. Cancer J 2014;20:18-24. [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [PubMed]
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 2010;464:431-5. [PubMed]
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 2010;140:209-21. [PubMed]
- Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 2011;29:2904-9. [PubMed]
- Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011;305:2327-34. [PubMed]
- Hodi FS, Friedlander P, Corless CL, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol 2008;26:2046-51. [PubMed]
- Carvajal RD, Hamid O, Antonescu CR. Selecting patients for KIT inhibition in melanoma. Methods Mol Biol 2014;1102:137-62. [PubMed]
- Woodman SE, Trent JC, Stemke-Hale K, et al. Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther 2009;8:2079-85. [PubMed]
- Zhu Y, Si L, Kong Y, et al. Response to sunitinib in Chinese KIT-mutated metastatic mucosal melanoma. J Clin Oncol 2009;27:e20017.
- Minor DR, Kashani-Sabet M, Garrido M, et al. Sunitinib Therapy for Melanoma Patients with KIT Mutations. Clinical Cancer Research 2012;18:1457-63. [PubMed]
- Kluger HM, Dudek AZ, McCann C, et al. A phase 2 trial of dasatinib in advanced melanoma. Cancer 2011;117:2202-8. [PubMed]
- Cho JH, Kim KM, Kwon M, et al. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Invest New Drugs 2012;30:2008-14. [PubMed]
- Agency EM. Summary of product characteristics: Tafinlar 2013 [cited 14.02.2014]. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002604/WC500149671.pdf
- Agency EM. Summary of product characteristics: Zelboraf 2013 [14.02.2014]. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002409/WC500124317.pdf
- Zimmer L, Livingstone E, Hillen U, et al. Panniculitis With Arthralgia in Patients With Melanoma Treated With Selective BRAF Inhibitors and Its Management. Arch Dermatol 2012;148:357-61. [PubMed]
- Anker CJ, Ribas A, Grossmann AH, et al. Severe liver and skin toxicity after radiation and vemurafenib in metastatic melanoma. J Clin Oncol 2013;31:e283-7. [PubMed]
- Regnier-Rosencher E, Lazareth H, Gressier L, et al. Acute kidney injury in patients with severe rash on vemurafenib treatment for metastatic melanomas. Br J Dermatol 2013;169:934-8. [PubMed]
- Iddawela M, Crook S, George L, et al. Safety and efficacy of vemurafenib in end stage renal failure. BMC Cancer 2013;13:581. [PubMed]
- Wolf SE, Meenken C, Moll AC, et al. Severe pan-uveitis in a patient treated with vemurafenib for metastatic melanoma. BMC Cancer 2013;13:561. [PubMed]
- Sandhu SS, Ling C, Lim L, et al. Vemurafenib (B-RAF inhibitor) associated uveitis in patients with metastatic cutaneous melanoma. Clin Exp Ophth 2012;40:118.
- Joshi L, Karydis A, Gemenetzi M, et al. Uveitis as a Result of MAP Kinase Pathway Inhibition. Case Rep Ophthalmol 2013;4:279-82. [PubMed]
- Klein O, Ribas A, Chmielowski B, et al. Facial palsy as a side effect of vemurafenib treatment in patients with metastatic melanoma. J Clin Oncol 2013;31:e215-7. [PubMed]
- Wisler JA, Afshari C, Fielden M, et al. Raf inhibition causes extensive multiple tissue hyperplasia and urinary bladder neoplasia in the rat. Toxicol Pathol 2011;39:809-22. [PubMed]
- Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: case report. Pharmacotherapy 2013;33:e43-4. [PubMed]
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010;464:427-30. [PubMed]
- Hall-Jackson CA, Eyers PA, Cohen P, et al. Paradoxical activation of Raf by a novel Raf inhibitor. Chemistry & biology 1999;6:559-68. [PubMed]
- Callahan MK, Rampal R, Harding JJ, et al. Progression of RAS-mutant leukemia during RAF inhibitor treatment. N Engl J Med 2012;367:2316-21. [PubMed]
- Cebon JS, Flaherty K, Weber JS, et al. Comparison of BRAF inhibitor (BRAFi)-induced cutaneous squamous cell carcinoma (cuSCC) and secondary malignancies in BRAF mutation-positive metastatic melanoma (MM) patients (pts) treated with dabrafenib (D) as monotherapy or in combination with MEK1/2 inhibitor (MEKi) trametinib (T). J Clin Oncol 2013;3:abstr 9016.
- Lacouture ME, Duvic M, Hauschild A, et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist 2013;18:314-22. [PubMed]
- Zimmer L, Vaubel J, Livingstone E, et al. Side effects of systemic oncological therapies in dermatology. J Dtsch Dermatol Ges 2012;10:475-86. [PubMed]
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol 2012;67:1265-72. [PubMed]
- Anforth R, Fernandez-Peñas P, Long GV. Cutaneous toxicities of RAF inhibitors. Lancet Oncol 2013;14:e11-8. [PubMed]
- Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 2012;366:207-15. [PubMed]
- Oberholzer PA, Kee D, Dziunycz P, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol 2012;30:316-21. [PubMed]
- Anforth RM, Blumetti TC, Kefford RF, et al. Cutaneous manifestations of dabrafenib (GSK2118436): a selective inhibitor of mutant BRAF in patients with metastatic melanoma. Br J Dermatol 2012;167:1153-60. [PubMed]
- Holderfield M, Lorenzana E, Weisburd B, et al. Vemurafenib Cooperates with HPV to Promote Initiation of Cutaneous Tumors. Cancer Res 2014;74:2238-45. [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [PubMed]
- Dummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemurafenib therapy. N Engl J Med 2012;366:480-1. [PubMed]
- Monfort JB, Pages C, Schneider P, et al. Vemurafenib-induced neutrophilic panniculitis. Melanoma Res 2012;22:399-401. [PubMed]
- Sinha R, Edmonds K, Newton-Bishop JA, et al. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanoma: practical advice on diagnosis, prevention and management of the main treatment-related skin toxicities. Br J Dermatol 2012;167:987-94. [PubMed]
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: An emergent adverse effect of variable severity. Dermatol Online J 2013;19:16. [PubMed]
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol 2013;24:1691-7. [PubMed]
- Degen A, Volker B, Kapp A, et al. Erythema nodosum in a patient undergoing vemurafenib therapy for metastatic melanoma. Eur J Dermatol 2013;23:118. [PubMed]
- Rinderknecht JD, Goldinger SM, Rozati S, et al. RASopathic skin eruptions during vemurafenib therapy. PLoS One 2013;8:e58721. [PubMed]
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in advanced melanoma patients undergoing selective BRAF inhibition. J Clin Oncol 2012;30:2375-83. [PubMed]
- Schmitt L, Schumann T, Inhoff O, et al. Eruptive Nevi Mimicking Wart-Like Lesions under Selective BRAF Inhibition in a 37-Year-Old Female Melanoma Patient. Case Rep Dermatol 2013;5:69-72. [PubMed]
- Dalle S, Poulalhon N, Thomas L. Vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine 2011;365:1448-9; author reply 50. [PubMed]
- Debarbieux S, Dalle S, Depaepe L, et al. Second primary melanomas treated with BRAF blockers: study by reflectance confocal microscopy. Br J Dermatol 2013;168:1230-5. [PubMed]
- Haenssle HA, Kraus SL, Brehmer F, et al. Dynamic changes in nevi of a patient with melanoma treated with vemurafenib: importance of sequential dermoscopy. Arch Dermatol 2012;148:1183-5. [PubMed]
- Zimmer L, Haydu LE, Menzies AM, et al. Incidence of New Primary Melanomas After Diagnosis of Stage III and IV Melanoma. J Clin Oncol 2014;32:816-23. [PubMed]
- Sinha R, Lecamwasam K, Purshouse K, et al. Toxic Epidermal Necrolysis in a patient receiving Vemurafenib for treatment of metastatic malignant melanoma. Br J Dermatol 2014;170:997-9. [PubMed]
- Wenk KS, Pichard DC, Nasabzadeh T, et al. Vemurafenib-induced DRESS. JAMA Dermatol 2013;149:1242-3. [PubMed]
- Pattanaprichakul P, Tetzlaff MT, Lapolla WJ, et al. Sweet syndrome following vemurafenib therapy for recurrent cholangiocarcinoma. J Cutan Pathol 2014;41:326-8. [PubMed]
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA dermatology 2013;149:855-7. [PubMed]
- Ducassou A, David I, Delannes M, et al. Radiosensitization induced by vemurafenib. Cancer Radiother 2013;17:304-7. [PubMed]
- Peuvrel L, Ruellan AL, Thillays F, et al. Severe radiotherapy-induced extracutaneous toxicity under vemurafenib. Eur J Dermatol 2013;23:879-81. [PubMed]
- Satzger I, Degen A, Asper H, et al. Serious skin toxicity with the combination of BRAF inhibitors and radiotherapy. J Clin Oncol 2013;31:e220-2. [PubMed]
- Rompoti N, Schilling B, Livingstone E, et al. Combination of BRAF Inhibitors and Brain Radiotherapy in Patients With Metastatic Melanoma Shows Minimal Acute Toxicity. J Clin Oncol 2013;31:3844-5. [PubMed]
- Harding JJ, Barker CA, Carvajal RD, et al. Cutis Verticis Gyrata in Association With Vemurafenib and Whole-Brain Radiotherapy. J Clin Oncol 2014;32:e54-6. [PubMed]
- Taylor SR, Isa H, Joshi L, et al. New developments in corticosteroid therapy for uveitis. Ophthalmologica 2010;224 Suppl 1:46-53. [PubMed]
- Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 2013;14:249-56. [PubMed]
- Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol 2013;31:482-9. [PubMed]
- Catalanotti F, Solit DB, Pulitzer MP, et al. Phase II trial of MEK inhibitor selumetinib (AZD6244, ARRY-142886) in patients with BRAFV600E/K-mutated melanoma. Clin Cancer Res 2013;19:2257-64. [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107-14. [PubMed]
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol 2012;13:782-9. [PubMed]
- Drugs.com. Mekinist Information from Drugs.com 2013-2014 [cited 14.02.2014]. Available online: http://www.drugs.com/sfx/mekinist-side-effects.html
- Moreno Garcia V, Thavasu P, Blanco Codesido M, et al. Association of creatine kinase and skin toxicity in phase I trials of anticancer agents. Br J Cancer 2012;107:1797-800. [PubMed]
- Chen X, Schwartz GK, DeAngelis LM, et al. Dropped head syndrome: report of three cases during treatment with a MEK inhibitor. Neurology 2012;79:1929-31. [PubMed]
- Boasberg PD, Redfern CH, Daniels GA, et al. Pilot study of PD-0325901 in previously treated patients with advanced melanoma, breast cancer, and colon cancer. Cancer Chemother Pharmacol 2011;68:547-52. [PubMed]
- Balagula Y, Barth Huston K, Busam KJ, et al. Dermatologic side effects associated with the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886). Invest New Drugs 2011;29:1114-21. [PubMed]
- Schad K, Baumann Conzett K, Zipser MC, et al. Mitogen-activated protein/extracellular signal-regulated kinase kinase inhibition results in biphasic alteration of epidermal homeostasis with keratinocytic apoptosis and pigmentation disorders. Clin Cancer Res 2010;16:1058-64. [PubMed]
- Robert C, Soria JC, Spatz A, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 2005;6:491-500. [PubMed]
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol 2013;169:172-6. [PubMed]
- Drugs.com. Gleevec Information from Drugs.com 2013 [20.02.2014]. Available online: http://www.drugs.com/pro/gleevec.html
- Drugs.com. Sprycel Information from Drugs.com 2013 [20.02.2014]. Available online: http://www.drugs.com/pro/sprycel.html
- Drugs.com. Tasigna Information from Drugs.com 2013 [20.02.2014]. Available online: http://www.drugs.com/pro/tasigna.html
- Drugs.com. Sutent Information from Drugs.com 2013 [20.02.2014]. Available online: http://www.drugs.com/pro/sutent.html
- Brazzelli V, Grasso V, Borroni G. Imatinib, dasatinib and nilotinib: a review of adverse cutaneous reactions with emphasis on our clinical experience. J Eur Acad Dermatol Venereol 2013;27:1471-80. [PubMed]
- Tsao AS, Kantarjian H, Cortes J, et al. Imatinib mesylate causes hypopigmentation in the skin. Cancer 2003;98:2483-7. [PubMed]
- Arora B, Kumar L, Sharma A, et al. Pigmentary changes in chronic myeloid leukemia patients treated with imatinib mesylate. Ann Oncol 2004;15:358-9. [PubMed]
- Etienne G, Cony-Makhoul P, Mahon FX. Imatinib mesylate and gray hair. N Engl J Med 2002;347:446. [PubMed]
- Valeyrie L, Bastuji-Garin S, Revuz J, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukemias: a prospective study of 54 patients. J Am Acad Dermatol 2003;48:201-6. [PubMed]
- Fraunfelder FW, Solomon J, Druker BJ, et al. Ocular side-effects associated with imatinib mesylate (Gleevec). J Ocul Pharmacol Ther 2003;19:371-5. [PubMed]


